CBSE 12th Standard Chemistry Subject Case Study Questions 2021
By QB365 on 21 May, 2021
QB365 Provides the updated CASE Study Questions for Class 12 , and also provide the detail solution for each and every case study questions . Case study questions are latest updated question pattern from NCERT, QB365 will helps to get more marks in Exams
QB365 - Question Bank Software
CBSE 12th Standard Chemistry Subject Case Study Questions 2021
12th Standard CBSE
-
Reg.No. :
Chemistry
-
Read the passage given below and answer the following questions:
The adjective, 'crystalline' when applied to solids, implies an ideal crystal in which the structural units, termed as unit cells, are repeated regularly and indefinitely in three dimensions in space. The unit cell, containing at least one molecule, has a definite orientation and shape defined by the translational vectors, a, band c. The unit cell therefore has a definite volume, V that contains the atoms and molecules necessary for generating the crystal. Every crystal can be classified as a member of one of the seven possible crystal systems or crystal classes that are defined by the relationships between the individual dimensions, a, band c of the unit cell and between the individual angles, \(\alpha, \beta \ and \ \gamma\) of the unit cell. The structure of the given crystal may be assigned to one of the 7 crystal systems, to one of the 14 Bravais lattices, and to one of the 230 space groups. These uniquely define the possible ways of arranging atoms in a three-dimensional solid. Based on these observations, seven crystal systems were identified: triclinic, monoclinic, trigonal or rhombohedral, tetragonal, hexagonal, rhombic or orthorhombic and cubic.
The following questions are multiple choice questions. Choose the most appropriate answer :
(i) The crystal system of a compound with unit cell dimensions, a = 0.387 nm, b = 0.387 nm and c = 0.504 nm and \(\alpha, \beta \ =90^o\) and \(\gamma\) = 120° is(a) cubic (b) hexagonal (c) orthorhombic (d) rhombohedral. (ii) The unit cell with the structure given below represents __________crystal system.
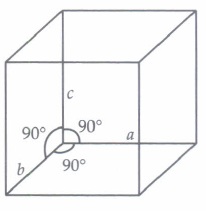
a) cubic (b) orthorhombic (c) tetragonal (d) trigonal (iii) In a triclinic crystal
\(\text { (a) } a=b=c, \quad \alpha=\beta=\gamma \neq 90^{\circ}\) \(\text { (b) } a \neq b=c, \quad \alpha=\beta=\gamma=90^{\circ}\) \(\text { (c) } a \neq b \neq c, \quad \alpha \neq \beta \neq \gamma \neq 90^{\circ}\) \(\text { (d) } a \neq b \neq c, \quad \alpha=\gamma=90^{\circ}, \beta \neq 90^{\circ}\) (iv) The unit cell with dimensions \(\alpha=\beta=\gamma=90^{\circ}, a=b \neq c \) is
(a) cubic (b) triclinic (c) hexagonal (d) tetragonal (a) -
Read the passage given below and answer the following questions:
The concentration of a solute is very important in studying chemical reactions because it determines how often molecules collide in solution and thus indirectly determine the rate of reactions and the conditions at equilibrium.
There are several ways to express the amount of solute present in a solution. The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution. Concentration can be expressed in terms of molarity, molality, parts per million, mass percentage, volume percentage, etc.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The molarity (in mol L-1) of the given solution will be(a) 1.56 (b) 1.89 (c) 0.263 (d) 1.44 (ii) Which of the following is correct relationship between mole fraction and molality?
\(\text { (a) } x_{2}=\frac{m M_{1}}{1+m M_{1}}\) \(\text { (b) } x_{2}=\frac{m M_{1}}{1-m M_{1}}\) \(\text { (c) } x_{2}=\frac{1+m M_{1}}{m M_{1}}\) \(\text { (d) } x_{2}=\frac{1-m M_{1}}{m M_{1}}\) (iii) Which of the following is temperature dependent?
(a) Molarity (b) Molality (c) Mole fraction (d) Mass percentage (iv) Which of the following is true for an aqueous solution of the solute in terms of concentration?
(a) 1 M = 1 m (b) 1M > 1m (c) 1M < 1 m (d) Cannot be predicted (a) -
Read the passage given below and answer the following questions:
Molar conductivity of ions are given as product of charge on ions to their ionic mobilities and Faraday's constant.
\(\lambda_{A^{n+}}=n \mu_{A^{n+}} F\) (here \(\mu\) is the ionic mobility of An+).
For electrolytes say AXBy, molar conductivity is given by
\(\lambda_{m\left(A_{x} B_{y}\right)}=x_{n} \mu_{A^{n+}} F+y_{m} \lambda_{A^{m}-F}\)Ions Ionic mobility K+ 7.616 x 10- 4 Ca2+ 12.33 x 10-4 Br- 8.09 x 10- 4 \(\mathrm{SO}_{4}^{2-}\) 16.58 x 10- 4 The following questions are multiple choice questions. Choose the most appropriate answer
(i) At infinite dilution, the equivalent conductance of CaSO4 is(a) 256 x 10-4 (b) 279 (c) 23.7 (d) 2.0 x 10- 8 (ii) If the degree of dissociation of CaSO4 solution is 10% then equivalent conductance of CaSO4 is
(a) 3.59 (b) 36.9 (c) 27.9 (d) 30.6 (iii) What is the unit of equivalent conductivity?
(a) ohm-1 cm2 eq-1 (b) ohm cm2eq-1 (c) ohm-1 cm eq-1 (d) ohm cm2 eq-1 (iv) If the molar conductance value of Ca2+ and Cl- at infinite dilution are 118.88 x 10-4 m2 mho mol-1 and 77.33 x 10-4 m 2 mho mol-1 respectively then the molar conductance of CaCl2 (in m2 mho mol-1) will be
(a) 120.18 x 10- 4 (b) 135 x 10-4 (c) 273.54 x 10-4 (d) 192.1 x 10-4 (a) -
Read the passage given below and answer the following questions :
The progress of the reaction, \(A \rightleftharpoons n B\) with time is represented in the following figure.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) What is the value of n?(a) 1 (b) 2 (c) 3 (d) 4 (ii) Find the-value of the equilibrium constant
(a) 0.6 M (b) 1.2M (c) 0.3M (d) 2.4M (iii) The initial rate of conversion of A will be
(a) 0.1 mol L-1 hr-1 (b) 0.2 mol L-1 hr-1 (c) 0.4 mol L-1 hr-1 (d) 0.8 mol L-1 hr-1 (iv) For the reaction, if \(\frac{d[B]}{d t}=2 \times 10^{-4}\) , value of \(-\frac{d[A]}{d t}\) will be
(a) 2 x 10- 4 (b) 10-4 (c) 4 x 10- 4 (d) 0.5 x 10- 4 (a) -
Read the passage given below and answer the following questions :
Adsorption depends on the nature of the adsorbent. The rough solid surface has more number of pores and adsorb more number of gases than the smooth surface. Most common adsorbents are silica gel, activated charcoaL The extent of adsorption also depends on the surface area of the solid. Specific surface area of an adsorbent is the surface area available for adsorption per gram of the adsorbent. The greater the surface area of the solid, the greater would be the adsorption. Charcoal is a more effective adsorbent than solid wood. Desorption is a process of removing an adsorbed substance from a surface on which it is absorbed.
Physisorption is non-specific and any gas can be adsorbed. But the gases which are easily liquefiable (e.g., NH3 , HCl, CO2 ) are adsorbed at a faster rate and to a large extent than the gases which are difficult to liquefy (e.g., H2 , O2, N2 ). It depends on the critical temperature. Higher the critical temperature of a gas, more easily liquefiable the gas is and more is the rate of adsorption. Chemisorption is specific in nature. Therefore, only those gases can be adsorbed which are capable of forming chemical bonds with the adsorbent.
The following questions are multiple choice questions. Choose the most appropriate answer :
(i) Select the correct statement regarding desorption.(a) It is done by cooling or by increasing the pressure applied. (b) It is done by cooling or by reducing the pressure applied. (c) It is done by heating or by reducing the pressure applied (d) It is done by heating or by increasing the pressure applied. (ii) Which of the following statements regarding the physical adsorption of a gas on surface of solid is not correct?
(a) On increasing temperature, adsorption increases continuously (b) Enthalpy changes are negative (c) It is non-specific in nature (d) It is reversible in nature (iii) At the same temperature and pressure, select the correct order of adsorption of the following gases on the same mass of charcoal.
(a) SO2 > CH4 > H2 (b) CH4 < SO2 < H2 (c) Hz > CH4 > SO2 (d) CH4 < H2 < SO2 (iv) Select the incorrect statement among the following.
(a) Physical adsorption occurs at a low temperature and chemisorption occurs at all temperature (b) In physisorption heat of adsorption is low while in chemisorption it is high (c) Chemisorption is irreversible and physisorption is reversible (d) Magnitude of chemisorption decreases with rise in temperature while physisorption increases with rise in temperature. (a) -
Read the passage given below and answer the following questions:
Under the normal conditions, noble gases are monoatomic and have closed shell electronic configuration. Lighter noble gases have low boiling points due to weak dispersion forces between the atoms and the absence of other interatomic interactions. Xenon, one of the important noble gas, forms a series of compounds with fluorine with oxidation number +2, +4 and +6. All xenon fluorides are strong oxidising agents. XeF4 reacts violently with water to give XeO3. The geometry of xenon compounds can be deduced by considering the total number of electron pairs in their valence shell.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Among noble gases (from He to Xe) only xenon reacts with fluorine to form stable xenon fluorides because xenon(a) has the largest size (b) has the lowest ionisation enthalpy (c) has the highest heat of vapourisation (d) is the most readily available noble gas. (ii) The structure of XeO3 is
(a) square planar (b) pyramidal (c) linear (d) T-shaped. (iii) In the preparation of compound of xenon, Bartlett had taken \(\mathrm{O}_{2}^{+} \mathrm{PtF}_{6}^{-}\) as a base compound. This is because
(a) both O2 and Xe have same size (b) both Xe and O2 have same electron gain enthalpy (c) both have almost same ionisation enthalpy (d) both Xe and O2 are gases. (iv) The oxidation state of xenon in XeO3 is
(a) +4 (b) +2 (c) +8 (d) +6 (a) -
Read the passage given below and answer the following questions:
The f-block elements are those in which the differentiating electron enters the (n -2) forbital. There are two series of f-block elements corresponding to filling of 4f and 5f-orbitals. The series of 4f- orbitals is called lanthanides. Lanthanides show different oxidation states depending upon stability of f0, f7 and f14 configurations, though the most common oxidation states is +3. There is a regular decrease in size of lanthanides ions with increase in atomic number which is known as lanthanide contraction.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The atomic numbers of three lanthanide elements X, Y and 2 are 65, 68 and 70 respectively, their Ln3+ electronic configuration is(a) 4f8, 4f11, 4f13 (b) 4f11, 4f8 , 4f13 (c) 4fo, 4f2, 4f11 (d) 4f3, 4f7, 4f9 (ii) Lanthanide contraction is observed in
(a) Gd (b) At (c) Xe (d) Te (iii) Name a member of the lanthanoid series which is well known to exhibit +4 oxidation state.
(a) Cerium (Z = 58) (b) Europium (Z = 63) (c) Lanthanum (Z = 57) (d) Gadolinium (Z = 64) (iv) Identify the incorrect statement among the following.
(a) Lanthanojd contraction is the accumulation of successive shrinkages. (b) The different radii of Zr and Hf due to consequence of the lanthanoid contraction. (c) Shielding power of 4f electrons is quite weak. (d) There is a decrease in the radii of the atoms or ions as one proceeds from La to Lu. (a) -
Read the passage given below and answer the following questions:
Coordination compounds are formulated and named according to the IUPAC system.
Few rules for naming coordination compounds are:
(I) In ionic complex, the cation is named first and then the anion.
(II) In the coordination entity, the ligands are named first and then the central metal ion.
(III) When more than one type of ligands are present, they are named in alphabetical order of preference without any consideration of charge.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The IUPAC name of the complex \(\left[\mathrm{Pt}\left(\mathrm{NH}_{3}\right)_{3} \mathrm{Br}\left(\mathrm{NO}_{2}\right) \mathrm{Cl}\right] \mathrm{Cl}\) is(a) triamminechlorobromonitroplatinum (IV) chloride (b) triamminebromonitrochloroplatinum (IV) chloride (c) triamminebromidochloridonitroplatinum (IV) chloride (d) triamminenitrochlorobromoplatinum (IV) chloride (ii) The lUPAC name of [Ni(CO)4] is
(a) tetracarbonylnickel (II) (b) tetracarbonylnickel (0) (c) tetracarbonylnickelate (II) (d) tetracarbonylnickelate (0). (iii) As per IUPAC nomenclature, the name of the complex \(\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\left(\mathrm{NH}_{3}\right)_{2}\right] \mathrm{Cl}_{3}\) is
(a) tetraaquadiamminecobalt (II) chloride (b) tetraaquadiamminecobalt (III) chloride (c) diamminetetraaquacobalt (II) chloride (d) diamminetetraaquacobalt (III) chloride (iv) Which of the following represents correct formula of dichloridobis( ethane-1, 2-diamine ) cobalt (III) ion?
(a) [CoCl2(en)]2+ (b) [Co(ONO)(NH3)5]SO4 (c) [Co(NO2)(NH3)4] (SO4)2 (d) [Co(NO)(NH3)4] (SO4)2 (a) -
Read the passage given below and answer the following questions:
Metal carbonyl is an example of coordination compounds in which carbon monoxide (CO) acts as ligand. These are also called homoleptic carbonyls. These compounds contain both \(\sigma\) and \(\pi\) character. Some carbonyls have metal-metal bonds. The reactivity of metal carbonyls is due to (i) the metal centre and (ii) the CO ligands. CO is capable of accepting an appreciable amount of electron density from the metal atom into their empty \(\pi\) or \(\pi\)* orbitals. These types ofligands are called \(\pi\)-accepter or \(\pi\)-acid ligands. These interactions increases the Δo value.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) What is the oxidation state of metal in [ Mn2(CO)10] ?(a) +1 (b) -1 (c) +2 (d) 0 (ii) Among the following metal carbonyls, the C - O bond order is lowest in
(a) \(\left[\mathrm{Mn}(\mathrm{CO})_{6}\right]^{+}\) (b) \(\left[\mathrm{Fe}(\mathrm{CO})_{5}\right]\) (c) \(\left[\mathrm{Cr}(\mathrm{CO})_{6}\right]\) (d) \(\left[\mathrm{V}(\mathrm{CO})_{6}\right]^{-}\) (iii) The oxidation state of cobalt in K [CO(CO)4] is
(a) +1 (b) +3 (c) -1 (d) 0 (iv) Structure of decacarbonyl manganese is
(a) trigonal bipyramidial (b) octahedral (c) tetrahedral (d) square pyramidal. (a) -
Read the passage given below and a.9swer the following questions:
A chlorocompound (A) on reduction with Zn-Cu and ethanol gives the hydrocarbon (B) with five carbon atoms. When (A) is dissolved in dry ether and treated with sodium metal it gave 2,2,5,5 tetramethylhexane. The treatment of (A) with alcoholic KCN gives compound (C).
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) The compound (A) is(a) 1-chloro- 2, 2-dimethylpropane (b) 1-chloro- 2, 2-dimethyl butane (c) 1-chloro-2-methyl butane (d) 2-chloro-2-methyl butane. (ii) The reaction of (C) with Na, C2H5OH gives
(a) (CH3)3C CH2CONH2 (b) (CH3)3C NH2 (c) (CH)3C CH2CH2NH2 (d) (CH3)2CHCH2NH2 (iii) The reaction of (C) with Na, C2H5OH is called
(a) Gilman reaction (b) Mendius reaction (c) Grooves process (d) Swart's reaction. (iv) Compound (B) is
(a) n-pentane (b) 2, 2-dimethylpropane (c) 2-methylbutane (d) none of these. (a) -
Read the passage given below and answer the following questions:
Although chlorobenzene is inert to nucleophilic substitution, however it gives quantitative yield of phenol when heated with aq. NaOH at high temperature and under high pressure. As far as electrophilic substitution in phenol is concerned the - OH group is an activating group, hence, its presence enhances the electrophilic substitution at o- and p-positions.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Conversion of chlorobenzene into phenol involves(a) modified SN1 mechanism (b) modified SN2 mechanism (c) both (a) and (b) (d) elimination-addition mechanism. (ii) Phenol undergoes electrophilic substitution more readily than benzene because
(a) the intermediate carbo cation is a resonance hybrid of more resonating structures than that from benzene (b) the intermediate is more stable as it has positive charge on oxygen, which can be better accommodated than on carbon (c) in one of the canonical structures, every atom (except hydrogen) has complete octet (d) the -OH group is o, p-directing which like all other o, p-directing group, is activating. (iii) Phenol on treatment with excess of conc. HNO3 gives
(a) o-nitrophenol (b) p-nitrophenol (c) o-and p-nitrophenol (d) 2, 4, 6-trinitrophenol (iv) The major product of the following reaction is
(a) -
Read the passage given below and answer the following questions:
Williamson's synthesis is used for the preparation of symmetrical as well as unsymmerical ether. It is SN2 reaction mechanism. In Williamson's synthesis, 1° alkyl halide are used for preparation of ethers because 2° and 3°alkyl halide give alkene: Ethers are cleaved by hydrogen halides to alcohol and alkyl halide where alkyl halide is corresponding to that alkyl which has less number of carbon atom (it is because ofless steric hindrance). In polar media unsymmetrical ether like tertiary butyl ethyl ether gives ethyl alcohol and tertiary butyl halide as reaction proceeds via carbocation.
In these questions (i-iv), a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
(i) Assertion: Rate of reaction of alkyl halide in Williamson's synthesis reaction is 1°RX> 2°RX > 3°RX.
Reason: It is a type of bimolecular substitution reaction (SN2).
(ii) Assertion: t-Butyl methyl ether is not prepared by the reaction of t-butyl bromide with sodium methoxide.
Reason: Sodium methoxide is a weak nucleophile.
(iii) Assertion: When isopropyl bromide is treated with sodium isopropoxide, di-isopropyl ether is obtained as a major product. .
Reason: With secondary alkyl halides, both substitution and elimination occur.
(iv) Assertion: Both symmetrical arid unsymmetrical ethers can be prepared by Williamson's synthesis.
Reason: Williamson's synthesis is an example of nucleophilic substitution reaction.(a) -
Read the passage given below and answer the following questions :
When an aldehyde with no a-hydrogen reacts with concentrated aqueous NaOH, half the aldehyde is converted to carboxylic acid salt and other half is converted to an alcohol. In other words, half of the reactant is oxidized
and other half is reduced. This reaction is known as Cannizzaro reaction
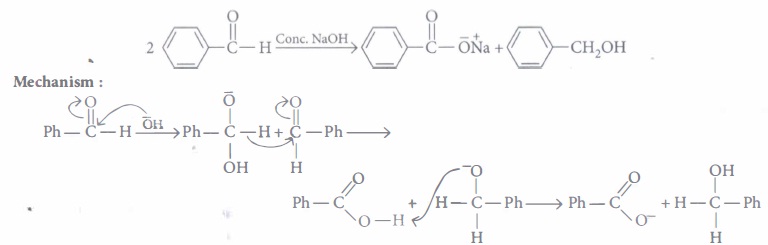
The following questions are multiple choice questions. Choose the most appropriate answer :
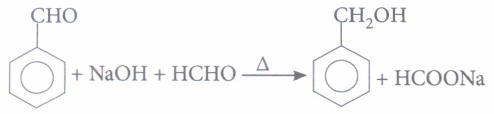
(i) A mixture of benzaldehyde and formaldehyde on heating with aqueous NaOH solution gives(a) benzyl alcohol and sodium formate (b) sodium benzoate and methyl alcohol (c) sodium benzoate and sodium formate (d) benzyl alcohol and methyl alcohol. (ii) Which of the following compounds will undergo Cannizzaro reaction?
(a) CH3CHO (b) CH3COCH3 (c) C6H5CHO (d) C6H5CH2CHO (iii) Trichloroacetaldehyde is subjected to Cannizzaro's reaction by using NaOH. The mixture of the products contains sodium trichloroacetate ion and another compound. The other compounds is
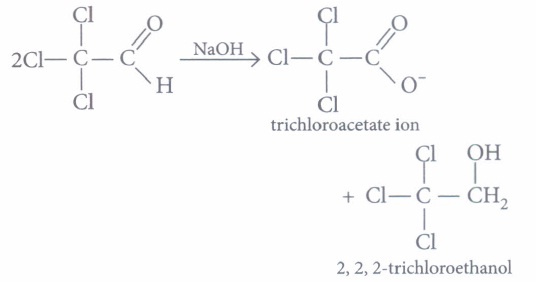
(a) 2, 2, 2-trichloroethanol (b) trichloromethanol (c) 2, 2, 2-trichloropropanol (d) chloroform (iv) Which of the following reaction will not result in the formation of carbon-carbon bonds?
(a) Cannizzaro reaction (b) Wurtz reaction (c) Reimer- Tiemann reaction (d) Friedel-Crafts acylation (a) -
Read the passage given below and answer the following questions:
When the mixture contains the three amine salts (1°, 2° and 3°) along with quaternary salt, it is distilled with KOH solution. The three amines distill, leaving the quaternary salt unchanged in the solution. Then the mixture of amines is separated by fractional distillation, Hinsbergs method and Hoffmann's method.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) Hinsberg reagent is(a) aliphatic sulphonyl chloride (b) phthalamide (c) aromatic sulphonyl chloride (d) anhydrous ZnCl2 + conc. HCl. (ii) Primary amine with Hinsberg's reagent forms
(a) N-alkyl benzene sulphonamide soluble in KOH solution (b) N-alkyl benzene sulphonamide insoluble in KOH solution (c) N, N-dialkyl benzene sulphonamide soluble in KOH solution (d) N, N-dialkyl benzene sulphonamide insoluble in KOH solution. (iii) To separate amines in a mixture Hoffmann's method is used. The Hoffmann's reagent is
(a) benzenesulphonyl chloride (b) diethyloxalate (c) benzeneisocyanide (d) p-toulenesulphonic acid. (iv) 3o amines with Hinsberg's reagent give
(a) no reaction (b) product which is same as that of 10 amine (c) product which is same as that of 2° amine (d) products which is a quaternary salt. (a) -
Read the passage given below and answer the following questions:
Pentose and hexose undergo intramolecular hemiacetal or hemiketal formation due to combination of the -OH group with the carbonyl group. The actual structure is either of five or six membered ring containing an oxygen atom. In the free state all pentoses and hexoses exist in pyranose form (resembling pyran). However, in the combined state some of them exist as five membered cyclic structures, called furanose (resembling furan).

The cyclic structure of glucose is represented by Haworth structure:
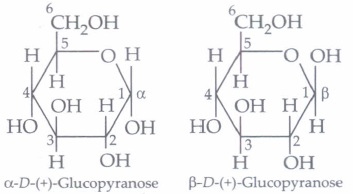
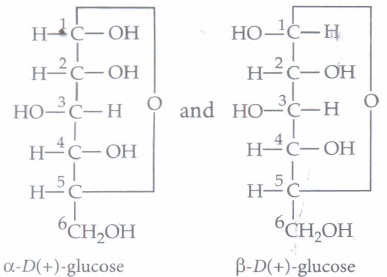
\(\alpha \) and \(\beta\) -D-glucose have different configuration at anomeric (C-l) carbon atom, hence are called anomers and the C-l carbon atom is called anomeric carbon (glycosidic carbon).
The six membered cyclic structure of glucose is called pyranose structure.
The following questions are multiple choice questions. Choose the most appropriate answer:
(i) \(\alpha\) -D(+)-glucose and \(\beta\) -D( +)glucose are(a) enantiomers (b) conformers (c) epimers (d) anomers (ii) The following carbohydrate is
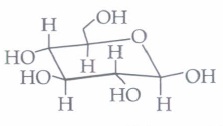
(a) a ketohexose (b) an aldohexose (c) an \(\alpha \)-furanose (d) an \(\alpha \)-pyranose (iii) In the following structure,
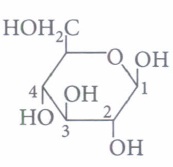
anomeric carbon is(a) C-l (b) C-2 (c) C-3 (d) C-4 (iv) The term anomers of glucose refers to
(a) isomers of glucose that differ in configurations at carbons one and four (C-l and C-4) (b) a mixture of (D)-glucose and (L)-glucose (c) enantiomers of glucose (d) isomers of glucose that differ in configuration at carbon one (C-l). (a)
*****************************************
CBSE 12th Standard Chemistry Subject Case Study Questions 2021 Answer Keys
-
(i) (b): For hexagonal crystal system, \(a=b \neq c\) and \(\alpha=\beta=90^{\circ}, \gamma=120^{\circ}\)
(ii) (a): Here, \(a=b=c ; \alpha=\beta=\gamma=90^{\circ}\) It belongs to cubic system.
(iii) (c)
(iv) (d): For tetragonal crystal system \(a=b \neq c \text { and }\)\(\alpha=\beta=\gamma=90^{\circ}\) -
(i) (d) : Density of solution = 1.202 g/mL
Volume of solution = \(\frac{100 \mathrm{~g}}{1.202 \mathrm{~g} / \mathrm{mL}}=83.2 \mathrm{~mL}\)
Molarity = \(\frac{n_{\mathrm{KI}}}{\text { Volume of solution in } \mathrm{L}}\)
\(=\frac{0.120 \mathrm{~mol}}{0.0832 \mathrm{~L}}=1.4423 \mathrm{~mol} \mathrm{~L}^{-1}\)
(ii) (a): \(x_{2}=\frac{n_{2}}{n_{1}+n_{2}} ; x_{1}=\frac{n_{1}}{n_{1}+n_{2}} ; \frac{x_{2}}{x_{1}}=\frac{n_{2}}{n_{1}}\)
\(\frac{x_{2}}{x_{1}}=\frac{m_{2} / M_{2}}{m_{1} / M_{1}}=\frac{m_{2}}{m_{1}} \times \frac{M_{1}}{M_{2}}\) ...(i)
Molality = \(\frac{n_{2}}{m_{1}}=\frac{m_{2}}{M_{2} \times m_{1}}\) ...(ii)
From(i) and (ii), m = \(\frac{x_{2}}{x_{1}} \times \frac{1}{M_{1}} ; x_{1}=1-x_{2}\)
Hence. x2 = \(\frac{m M_{1}}{1+m M_{1}}\)
(iii) (a) : Mass does not depend on temperature while volume does. Hence, molarity depends on temperature.
(iv) (b): 1M solution contains 1 mole of solute in less than 1000 g of the solvent whereas 1 m solution has 1 mole of the solute in 1000 g of the solvent. -
(i) (b) : Equivalent conductance of CaSO4 :
\(\Lambda_{\mathrm{CaSO}_{4}}^{\infty}=\lambda_{\mathrm{Ca}^{2+}}^{\infty}+\lambda_{\mathrm{SO}_{4}^{2-}}^{\infty}\)
\(\lambda_{\mathrm{Ca}^{2+}}^{\infty}=\left(\mu_{\mathrm{Ca}^{2+}}\right) F ; \lambda_{\mathrm{SO}_{4}^{2-}}^{\infty}=\left(\mu_{\mathrm{SO}_{4}^{2-}}\right) F\)
\(\mu_{\mathrm{Ca}^{2+}}\) and \(\mu_{\mathrm{SO}_{4}^{2-}}\) - are ionic mobilities.
\(\Lambda_{\mathrm{CaSO}_{4}}^{\infty}=F(12.33+16.58) \times 10^{-4}\)
= 96500 x 10- 4 x 28.91 = 279
(ii) (c) : \(\alpha=\frac{\Lambda_{C}}{\Lambda^{\infty}} \Rightarrow 0.1=\frac{\Lambda_{C}}{279} \Rightarrow \Lambda_{C}=27.9\)
(iii) (a)
(iv) (c) : \(\Lambda_{m\left(\mathrm{CaCl}_{2}\right)}^{\circ}=\lambda_{\mathrm{Ca}^{2+}}^{\circ}+2 \lambda_{\mathrm{Cl}^{-}}^{\circ}\)
= (118.88 x 10- 4 ) + 2(77.33 x 10- 4 )
= 273.54 x 10-4 m2 mho mol-1 -
(i) (b) : According to the figure, in the given time of 4 hours (1 to 5) concentration of A falls from 0.5 to 0.3 M, while in the same time concentration of B increases from 0.2 to 0.6 M.
Decrease in concentration of A in 4 hours
= 0.5 - 0.3 = 0.2 M
Increase in concentration of B in 4 hours
= 0.6 - 0.2 = 0.4 M
Thus, increase in concentration of B in a given time is twice the decrease in concentration of A. Thus, n = 2
(ii) (b) : \(K=\frac{[B]^{2}}{[A]}=\frac{(0.6)^{2}}{0.3}=1.2 \mathrm{M}\)
(iii) (a) : From t = 0 to t = 1 hr,
For A, dx = 0.6 - 0.5 = 0.1 mol L-1
\(\therefore\) Initial rate of conversion of \(A=\frac{d x}{d t}\)
\(=\frac{0.1 \mathrm{~mol} \mathrm{~L}^{-1}}{1 \mathrm{hr}}=0.1 \mathrm{~mol} \mathrm{~L}^{-1} \mathrm{hr}^{-1}\)
(iv) (b) : \(A \rightleftharpoons 2 B\)
\(-\frac{d[A]}{d t}=+\frac{1}{2} \frac{d[B]}{d t}=\frac{1}{2} \times 2 \times 10^{-4}=10^{-4}\) -
(i) (c) : Desorption is done by heating or by reducing the pressure applied.
(ii) (a) : Physisorption is exothermic in nature. Therefore, according to Le Chatelier's principle, it occurs readily at low temperature and decreases with increase in temperature. Bonds between surface and adsorbate are weak so when temperature is increased the bonds break easily, so rate will decrease on increasing temperature.
(iii) (a) : Higher the critical temperature of a gas, greater is the amount of gas adsorbed. Critical temperature (in Kelvin) of the gases :
\(\begin{array}{l} \mathrm{H}_{2}<\mathrm{CH}_{4}<\mathrm{SO}_{2} \\ 33.2 \quad 190.6 \quad 430.3 \end{array}\)
(iv) (d) : Chemisorption first increases with increase of temperature. Physisorption decreases with rise in temperature. -
(i) (b)
(ii) (b) :
(iii) (c)
(iv) (d) : \(\mathrm{XeO}_{3} \Rightarrow x+(-2) \times 3=0 \Rightarrow x=+6\) -
(i) (a):Terbium (65), 4f8; Dysprosium (Dy), 4f9; Ytterbium (Yb), 4f13.
(ii) (a)
(iii) (a)
(iv) (b): The almost identical radii of Zr (160 pm) and Hf(159 pm), a consequence of lanthanoid contraction. -
(i) (c): Ligands are named in alphabetical order irrespective of their charge.
(ii) (b)
(iii) (d)
(iv) (d) -
(i) (d): Oxidation state of Mn in [Mn2(CO)10] is zero.
(ii) (d): In \(\left[\mathrm{V}(\mathrm{CO})_{6}\right]^{-}\), the anionic carbonyl complex can delocalise more electron density to antibonding \(\pi\)-orbital (d\(\pi\)-p\(\pi\) back bonding) of CO and thus lowers the bond order.
(iii) (c): K[CO(CO)4]
+ 1 + (x) + 4(0) = 0 or x = -1
(iv) (d): Mn2(CO)10 is made up of two square pyramidal Mn(CO)5 units joined by Mn - Mn bond. -
(iii) (b)
-
(i) (d)
-
(i) (a): Williamson's synthesis occurs by SN2 mechanism and primary alkyl halides are most reactive in SN2 reactions.
(ii) (c): Sodium methoxide is a strong nucleophile. In presence of a strong base, i.e., sodium methoxide, t-butyl bromide undergoes dehyrohalogenation to form isobutylene.
(iii) (d): Since secondary and tertiary alkyl halides prefer to undergo elimination rather than substitution, therefore, even symmetrical ethers containing secondary and tertiary alkyl groups cannot be prepared in good yields by Williamson synthesis.
(iv) (b): Depending upon whether the alkyl halide and the alkoxide ion carry the same or different alkyl groups, both symmetrical and unsymmetrical ethers can be prepared by Williamsons synthesis. -
(i) (a): It is an example of cross Cannizzaro reaction where aromatic aldehyde gets reduced to alcohol and aliphatic aldehyde gets oxidised to its sodium salt (both aldehydes must not contain any \(\alpha\)-hydrogen).
(ii) (c)
(iii) (a): The Cannizzaro product of given reaction yields 2, 2, 2-trichloroethanol.
(iv) (a): C-C bond is not formed in Cannizzaro reaction while other reactions result in the formation of C-C bond. -
(i) (c)
(ii) (a): A primary amine forms N-alkylbenzene sulphonamidewhich because ofthe presence of an acidic hydrogen on the N-atom dissolves in aqueous KOH.
(iii) (b)
(iv) (a): Tertiary amine does not contain a replaceable hydrogen on the nitrogen atom. So, 3o amine does not react with Hinsberg's reagent. -
(i) (d): \(\alpha \)-D-(+)-glucose and \(\beta \) -D-(+)-glucose differ in configuration at C1 (U., anomeric or glycosidic carbon) and hence are called anomers.
(ii) (b): This structure is an example of pyranose and aldohexose. Here, the carbohydrate's structure is of the \(\beta \) -pyranose form.
(iii) (a) : C-1 is the anomeric carbon.
(iv) (d): Anomers are cyclic monosaccharides or glycosides that are epimers, differing from each other in the configuration at C-1, if they are aldoses or in the configuration at C- 2 if they are ketoses.






































