Class 10th Science - Acids, Bases and Salts Case Study Questions and Answers 2022 - 2023
By QB365 on 09 Sep, 2022
QB365 provides a detailed and simple solution for every Possible Case Study Questions in Class 10 Science Subject - Acids, Bases and Salts, CBSE. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
QB365 - Question Bank Software
Acids, Bases and Salts Case Study Questions With Answer Key
10th Standard CBSE
-
Reg.No. :
Science
-
The acidic behaviour of acids is due to the presence of hydrogenil(H+)ions in them. They produce hydrogen ions in the presence of water. Water is a polar solvent and this property of water helps in weakening the bond between the ions and makes them soluble. Hence, acids and bases produce ions in aqueous solutions.
It may be noted that a dry Hel gas or a solution of hydrogen chloride in organic, nonpolar solvents like toluene or benzene do not show acidic properties. This is because hydrogen chloride does not undergo ionization in toluene.
The reason why Hel splits into H+ and CI- ions in presence of water lies in the fact that water molecules, being polar, pull the H+ and Cl - ions apart and thus, the bond in Hel is broken
(i) Identify the wrong statement.(a) Higher the hydronium ion concentration, lower is the pH value (b) Universal indicator is used to judge how strong a given acid or base is (c) As the pH value increases from 7 to 14, it represents increase in H+ ion concentration in the solution (d) Value less than 7 on the pH scale represents an acidic solution (ii) If the pH of a solution is 8, then its [H+] ion is
(a) log 10- 8 (b) 108 (c) 10- 8 (d) 8 (iii) In terms of acidic strength, which one of the following is in the correct increasing order?
(a) Water < Acetic acid < Hydrochloric acid (b) Water < Hydrochloric acid < Acetic acid (c) Acetic acid < Water < Hydrochloric acid (d) Hydrochloric acid < Water < Acetic acid (iv) Which of the following compounds does not give H+ ions in aqueous solution?
(a) H3 PO4 (b) C2H5OH (c) H2CO3 (d) CH3COOH (v) Four solutions labelled as P, Q, Rand Shave pH values 1, 9, 3 and 13 respectively.
Which of the following statements about the given solutions is incorrect?(a) Solution P has higher concentration of hydrogen ions than solution R. (b) Solution Q has lower concentration of hydroxide ions than solution S. (c) Solutions P and Q will turn red litmus solution blue. (d) Solution P is highly acidic while solution Q is weakly basic. (a) -
A compound, X of sodium forms a white powder. It is a constituent of baking powder and is used in some antacids. When heated it gives a compound, Y which is anhydrous and absorbs water to become a hydrated salt. When this salt is kept in open air, it loses water molecules in a process called efflorescence. When dissolved in water it forms a strong base and a weak acid, Z.
(i) What is the compound, X?(a) NaHCO3 (b) Na2CO3 (c) NaOH (d) NaCl (ii) The compound, Y is
(a) NaHCO3 (b) Na2CO3 (c) Na2CO3 10H2O (d) NaCI (iii) What is the nature of the solution formed by dissolving Y in water?
(a) Alkaline (b) Acidic (c) Neutral (d) It remains insoluble (iv) Identify the compound, Z.
(a) CO2 (b) H2CO3 (c) NaOH (d) H2O (v) Sodium carbonate is a basic compound because it is a salt of a
(a) strong acid and strong base (b) weak acid and weak base (c) strong acid and weak base (d) weak acid and strong base (a) -
Sodium chloride obtained from sea water or from lakes contains many impurities such as sulphates of sodium and magnesium along with chlorides of calcium and magnesium. The chlorides of calcium and magnesium are particularly undesirable on account of their deliquescent nature.
For its purification, common salt is dissolved in minimum quantity of water to get a saturated solution from which insoluble impurities are filtered off. Then hydrogen chloride gas is passed through the saturated solution and the crystals of pure NaCl separate out. The soluble impurities remain in the mother liquor. The crystals are filtered, washed and dried.
(i) Select the correct statement regarding salt NaCl.(a) Pure NaCI is hygroscopic in nature (b) It is soluble in alcohol (c) Pure NaCI is not hygroscopic, it shows hygroscopic nature due to impurities (d) It is a brown crystalline solid (ii) Nature of aqueous solution of common salt is
(a) acidic (b) alkaline (c) basic (d) neutral (iii) In the given series of reactions, Y and Z respectively are
2
(a) NaHCO3 , NaOCl2 (b) NH4CI, Na2CO3 (c) Na2CO3 , NH4CI (d) Na2CO3, NaHCO3 (iv) Which of the following compounds is alkaline in aqueous medium?
(a) Na2CO3 (b) NaCI (c) H2CO3 (d) CuSO4 (v) Some statements regarding salt NaCI are given below
(I) It is prepared by chlor-alkali process
(II) It is a white crystalline substance
(III) It also exists in the form of rocks and is called rock salt
(IV) It is a neutral salt, pH value of NaCI is 7(a) II and III only (b) III and IV only (c) I and IV only (d) II, III and IV only (a) -
Chemically, Plaster of Paris (POP) is calcium sulphate hemihydrate, i.e., containing half molecule of water of crystallisation. It is represented by the formula, CaSO4 ·1/2H2O. Half molecule of water of crystallisation means that one water molecule is shared by two formula units of CaSO4. Hence, we also represent its formula as (CaSO4)2·H2O. The name, plaster of Paris, was given to this compound because for the first time, it was made from gypsum which was mainly found in Paris.
(i) The difference of water molecules in gypsum and plaster of Paris is(a) 5/2 (b) 2 (c) 1/2 (d) 3/2 (ii) Plaster of Paris hardens by
(a) giving off CO2 (b) changing into CaCO3 (c) combining with water (d) giving out water (iii) Which of the following statements is incorrect?
(a) Plaster of Paris is used to ornate designs on walls and ceilings (b) On heating gypsum above 373 K, CaSO4 is obtained (c) Dead burnt plaster is CaSO4 ·2H2O (d) Setting of plaster is due to its hydration into gypsum (iv) Select the incorrect statement with respect to gypsum
(a) It is slightly soluble in water (b) It is also known as alabaster (c) On heating gypsum at 373 K, it loses water molecules and becomes calcium sulphate hemihydrate (d) Chemical formula of gypsum is CaSO4 ·1/2H2O (v) Plaster of Paris is obtained by
(a) adding water to calcium sulphate. (b) adding sulphuric acid to calcium hydroxide (c) heating gypsum to a very high temperature (d) heating gypsum to 100° C (a) -
pH is quite useful to us in a number of ways in daily life. Some of its applications are:
Control of pH of the soil: Plants need a specific pH range for proper growth. The soil may be acidic, basic or neutral depending upon the relative concentration of H+ and OH-. The pH of any soil can be determined by using pH paper. If the soil is too acidic, it can be corrected by adding lime to it. If the soil is too basic, it can be corrected by adding organic manure which contains acidic materials
Regaining shine of a tarnished copper vessel by use of acids: A copper vessel gets tarnished due to formation of an oxide layer on its surface. On rubbing lemon on the vessel, the surface is cleaned and the vessel begins to shine again. This is due to the fact that copper oxide is basic in nature, which reacts with the acid (citric acid) present in lemon to form a salt (copper citrate) which is washed away with water. As a result, the layer of copper oxide is removed from the surface of the vessel and the shining surface is exposed
(i) When black copper oxide placed in a beaker is treated with dilute HCl, its colour changes to(a) white (b) dark red (c) bluish green (c) bluish green (d) no change (ii) P is an aqueous solution of acid and Q is an aqueous solution of base. When these two are diluted separately, then
(a) pH of P increases while that of Q decreases till neutralisation (b) pH of P decreases while that of Q increases till neutralisation (c) pH of both P and Q decrease (d) pH of both P and Q increase (iii) Which of the following acids is present in bee sting?
(a) Formic acid (c) Citric acid (b) Acetic acid (b) Acetic acid (d) Hydrochloric acid (iv) Sting of ant can be cured by rubbing the affected area with soap because
(a) it contains oxalic acid which neutralises the effect of formic acid (b) it contains aluminium hydroxide which neutralises the effect of formic acid (c) it contains sodium hydroxide which neutralises the effect of formic acid (d) none of these (v) The pH of soil X is 7.5 while that of soil Y is 4.5. Which of the two soils, should be treated with powdered chalk to adjust its pH?
(a) X only (b) Y only (c) Both X and Y (d) None of these (a) -
Baking powder produces carbon dioxide on heating, so it is used in cooking to make the batter spongy. Although, baking soda also produces CO2 on heating, but it is not used in cooking because on heating, baking soda produces sodium carbonate along with carbon dioxide. Sodium carbonate, thus, produced, makes the taste bitter. Baking powder is the mixture of baking soda and a mild edible acid. Generally, tartaric acid is mixed with baking soda to make baking powder. When baking powder is heated, NaHCO3 decomposes to give CO2 which makes bread and cake fluffy. Tartaric acid helps to remove bitter taste due to formation of sodium tartrate.
\(2 \mathrm{NaHCO}_{3}+ \ \ \mathrm{C}_{4} \mathrm{H}_{6} \mathrm{O}_{6} \quad \longrightarrow \quad 2 \mathrm{CO}_{2}+2 \mathrm{H}_{2} \mathrm{O}+\mathrm{Na}_{2} \mathrm{C}_{4} \mathrm{H}_{4} \mathrm{O}_{6}\)
Baking soda Tartaric acid Carbon dioxide Sodium tartrate
(i) On passing excess CO2 gas in aqueous solution of sodium carbonate, the substance obtained is(a) NaOH (b) NaHCO3 (c) Na2CO3 ·10H2O (d) Na2CO3 ·H2O (ii) When sodium hydrogen carbonate is added to acetic acid, it evolves a gas. Which of the following statements are true about the gas evolved?
(I) It turns lime water milky
(II) It extinguishes a burning splinter
(III) It dissolves in a solution of sodium hydroxide
(IV) It has a pungent odour(a) (I) and (II) (b) (I), (II) and (III) (c) (II), (III) and (IV) (d) (I) and (IV) (iii) Select the correct statement regarding sodium hydrogen carbonate.
(a) CO and CO2 are produced during the heating ofNaHCO3 (b) It is insoluble in water. (c) It is used in soda-acid fire extinguishers. (d) All of these (iv) Acetic acid was added to a solid X kept in a test tube. A colourless and odourless gas was evolved. The gas was passed through lime water which turned milky. It was concluded that
(a) solid X is sodium hydroxide and the gas evolved is CO2 (b) solid X is sodium bicarbonate and the gas evolved is CO2 (c) solid X is sodium acetate and the gas evolved is CO2 (d) solid X is sodium chloride and the gas evolved is CO2 (v) Which of the following statements are correct regarding baking soda?
(I) Baking soda is sodium hydrogen carbonate
(II) On heating, baking soda gives sodium carbonate
(III) It is used for manufacture of soap
(IV) It is an ingredient of baking powder(a) I and IV only (b) I, II and III only (c) I, II and IV only (d) I, II, III and I (a) -
Bleaching powder is also known as chloride of lime. It is a solid and yellowish white in colour. Bleaching powder can be easily identified by the strong smell of chlorine. When calcium hydroxide (slaked lime) reacts with chlorine, it gives calcium oxychloride (bleaching powder) and water is formed. Aqueous solution of bleaching powder is basic in nature. The material to be bleached is first passed through solution of NaOH to remove greasy matter. Then it is passed through aqueous solution of bleaching powder and very dil. HCI solution. HCI reacts with bleaching powder to liberate nascent oxygen which bleaches material.
(i) Bleaching powder is used as(a) bleaching agent in textile, paper and jute industry (b) disinfectant for water to make water free of germs (c) oxidising agent in many industries (d) all of these (ii) Bleaching powder is also known as
(a) calcium oxychloride (b) calcium hypochlorite (c) chloride of lime (d) all of these. (iii) Bleaching powder gives smell of chlorine because it
(a) is unstable (b) gives chlorine on exposure to atmosphere (c) is a mixture of chlorine and slaked lime (d) contains excess of chlorine (iv) Select the correct statement(s) regarding bleaching powder.
(a) It is pale yellow powder having smell of chlorine (b) It is sparingly soluble in water and gives milky suspension when dissolved in water (c) As bleaching powder gives nascent oxygen, it shows bleaching property (d) All of these (v) Identify the product 'X' in the given reaction \(\mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{Cl}_{2} \longrightarrow X+\mathrm{H}_{2} \mathrm{O}\)
(a) CaOCl2 (b) CaCl2 (c) Ca(CIO3 )2 (d) CaCO3 (a) -
The preparation of washing soda is carried out through following steps:
Step-I: Manufacture of sodium hydrogen carbonate:\(\mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}+\mathrm{NH}_{3}+\mathrm{CO}_{2} \longrightarrow \ \mathrm{NaHCO}_{3}+ \ \mathrm{NH}_{4} \mathrm{Cl}\)
Sodium hydrogen carbonate
Step-II: Thermal decomposition of sodium hydrogen carbonate: When dry crystals of sodium hydrogen carbonate are heated strongly, they decompose to form anhydrous sodium carbonate (soda ash). \(2 \mathrm{NaHCO}_{3(s)} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3(s)}+\mathrm{CO}_{2(g)}+\mathrm{H}_{2} \mathrm{O}_{(g)}\)
Step-III: Recrystallisation of sodium carbonate: Sodium carbonate thus obtained is recrystallised to form crystals of washing soda.\(\mathrm{Na}_{2} \mathrm{CO}_{3(s)}+ 10 \mathrm{H}_{2} \mathrm{O}_{(l)} \longrightarrow \mathrm{Na}_{2} \mathrm{CO}_{3} \cdot 10 \mathrm{H}_{2} \mathrm{O}_{(s)}\)
Anhydrous Washing soda
sodium carbonate
(i) Some of the uses of washing soda are given below:
(I) It is used for removing permanent hardness of water
(II) It is used in glass industry
(III) It is used in paper industry
(IV) It is used in the manufacture of sodium compounds such as borax
Select the correct option regarding uses of washing soda(a) (I) and (II) only (b) (II) and (III) only (c) (II) and (IV) only (d) (I), (II), (III) and (IV) (ii) What products will be formed along with water when sodium carbonate reacts with dilute hydrochloric acid?
(a) CO and NaCI (b) Na and CO2 (c) NaCI and CO2 (d) Na and CO (iii) Chief raw materials for the manufacture of washing soda are
(a) sodium chloride, ammonia and limestone (b) ammonia, sodium hydrogen carbonate and copper sulphate (c) sodium hydroxide, calcium chloride and ammonia (d) calcium chloride, sodium chloride and copper sulphate. (iv) What is the action of sodium carbonate on litmus paper?
(a) Turns red litmus blue (b) Turns blue litmus red (c) No change on litmus (d) Both (a) and (b) (v) What products will be obtained when solution of sodium carbonate and slaked lime is heated?
(a) NaOH and CaCl2 (b) CaCO3 and NaOH (c) NaHCO3 and NaOH (d) NaCI and CaCO3 (a) -
"Indicator is a chemical compound which is added to the solution in very small amount to detect its acidic or basic nature:" As they show colour change in acidic and basic medium, they are also called acid-base indicators. In other words, "an acid-base indicator is that substance which possesses one colour in acidic medium and a different colour in alkaline medium:''
Indicators, basically, are coloured organic substances either extracted from plants (natural indicators) or synthesised in the laboratory (synthetic indicators). A few common acid base indicators are: Litmus, phenolphthalein, methyl orange etc. In addition to these there are some naturally occurring substances which have different smell in acidic and basic medium. These substances are called olfactory indicators.
(i) Which one of the following will turn red litmus blue?(a) Vinegar (b) Baking soda solution (c) Lemon juice (d) Soft drinks (ii) A solution turns blue litmus red. The pH of the solution is probably
(a) 8 (b) 10 (c) 12 (d) 6 (iii) A solution in test tube 'A' turns red litmus blue, evolves hydrogen gas on reaction with zinc and does not react with sodium carbonate. Whereas, solution in test tube 'B' turns blue litmus red, liberates hydrogen gas on reaction with zinc and evolves carbon dioxide gas with sodium carbonate. Identify 'A' and 'B'
(a) 'A' is an acid, 'B' is a base (b) 'A' is a base, 'B' is an acid (c) Both 'A' and 'B' are bases (d) Both 'A' and 'B' are acids (iv) Select the incorrect option
Indicator Colour in acidic medium Colour in basic medium (a) Litmus (Purple) Red Blue (b) Flower of hydrangea plant (Blue) Red Green (c) Red cabbage juice (Purple) Red or Pink Green (d) Turmeric Juice (Yellow) Yellow Reddish brown (v) Which one of the following can be used as an acid-base indicator by visually impaired student?
(a) Litmus (b) Turmeric (c) Vanilla essence (d) Methyl orange (a) -
Acids turn blue litmus red but have no effect on red litmus. Bases turn red litmus blue but have no effect on blue litmus. The sample in which phenolphthalein remains colourless while methyl orange changes to pink/ red are acids while the samples in which phenolphthalein colour changes to pink and methyl orange changes to yellow are bases. Some observations of different sample solutions in litmus, phenolphthalein and methyl orange indicator are given in the table.
Sample solution Red litmus solution Blue litmus solution Phenolphthalein indicator Methyl orange indicator
HCI No colour change Red Colourless Red/ Pink H2 SO4 No colour change Red Colourless Red/ Pink HNO3 No colour change Red Colourless Red/ Pink CH3COOH No colour change Red Colourless Red/ Pink NaOH Blue No colour change Pink Yellow Ca(OH)2 Blue No colour change Pink Yellow KOH Blue No colour change Pink Yellow Mg(OH)2 Blue No colour change Pink Yellow NH4 OH Blue No colour change Pink (Becomes colourless after sometime) Yellow (Becomes colourless after sometime) (i) Which of the following substances does not turn red litmus solution to blue?
(a) AI(OH)3 (b) Mg(OH)2 (c) H3 PO4 (d) NH4OH (ii) Phenolphthalein's colour in basic medium is ___________but in acid it is ___________ .
(a) pink, colourless (b) yellow, pink (c) pink, orange (d) blue, red (iii) Which of the following acids are edible?
(I) Citric acid
(II) Tartaric acid
(III) Hydrochloric acid
(IV) Carbonic acid.(a) (I) and (II) only (b) (1), (II) and (IV) only (c) (I), (II) and (III) only (d) (I), (II), (III) and (IV) (iv) The colour of methyl orange in neutral solution is
(a) red (b) orange (c) yellow (d) purple (v) Which of the following cannot act as an indicator?
(a) Methyl orange (b) Methyl chloride (c) Turmeric juice (d) Phenolphthalein (a) -
A girl met with an accident and his leg fractured. She went to orthopedics doctor for treatment. On examination, the doctor mixed the white power in water and applied to her leg along with the cotton and gauze. After a while, it turned into white, solid, hard mass. He said that it would support her fractured bone in the right position.
(i) After treatment, the doctor repacked the white powder back into moisture proof, airtight container. Why?
(a) The fungus growth will occur in open
(b) The powder would react to moisture and turn into solid mass
(c) The powder with react to sunlight and turn into solid mass
(d) To prevent the stealing of the powder as it is very expensive
(ii) What is 'white, solid hard mass' called as?
(a) Talcum powder (b) Paris of Plaster
(c) Plaster of Paris (d) Copper sulphate
(iii) The graph shows the porosity and expansion of plaster with respect to water content
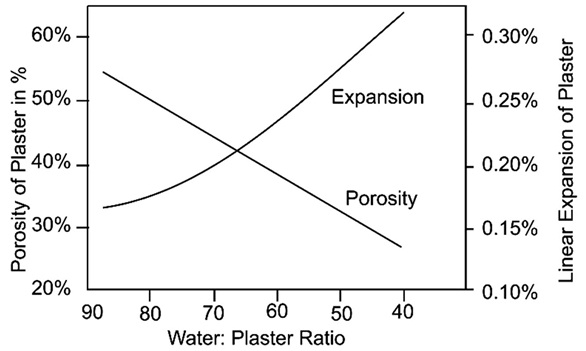
At what temperature, the reaction would occur?(a) 373K
(b) 673K (c) 273K (d) 573K (iv) The reaction involved in the formation of white mass is:
(a) Combustion
(b) Oxidation (c) Mineralisation (d) Crystallisation (v) Study the following reaction and choose the correct option:
\(\mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}+\frac{3}{2} \mathrm{H}_{2} \mathrm{O} \longrightarrow \mathrm{CaSO}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O}\)
(a) Reactant is calcium hemihydrate, product is Gypsum
(b) Reactant is Gypsum, product is calcium hemihydrate
(c) Reactant is Gypsum, product is calcium sulphate hemihydrate
(d) Reactant is calcium sulphate hemihydrate, product is Gypsum
(a) -
Ajay wanted his house to be white washed. He bought 10 kg of quicklime from the market. Before mixing all 10 kg, he took one beaker and took small quantity of quicklime in a beaker then he added some water, he observed that the water started boiling even when it was not being heated and he touch the beaker carefully. The beaker feels to be quite hot
(i) What is formed when water is added to quicklime.
(a) CaCO3 (b) CaO (c) Ca(OH)2 (d) NaOH
(ii) The nature of the product formed is:
(a) Acidic (b) Basic (c) Neutral (d) Both (a) and (b)
(iii) The chemical reaction between quicklime and water is characterised by :
(a) evolution of hydrogen gas
(b) formation of slaked lime precipitate
(c) change in temperature of mixture
(d) change in colour of the product
The chemical reaction between quicklime (CaO) and water is characterized by change in temperature of mixture. The reaction is exothermic (heat is liberated) and a hissing sound is heard. The product is slaked lime (calcium hydroxide).
(iv) Which of the following statements is correct about the above reaction based on your observations?
I. It is an endothermic reaction.
II. It is an exothermic reaction
III. The pH of the resulting solution will be more than seven.
IV. The pH of the resulting solution will be less than seven.
(a) I and II (b) II and III (c) I and IV (d) III and IV
It is an exothermic reaction because heat is given out. The resulting compound is Ca(OH)2 which is basic in nature. So the pH of the resulting solution will be more than seven.
(v) Which of the following is not an endothermic reaction ?
(a) CaCO3 → CaO + CO2
(b) 2H2O → 2H2 + O2
(c) 6CO2 + 6H2O → C6H12O6 + 6O2
(d) C6H12O6 + 6O2 → 6CO2 + 6H2O
During respiration, glucose combines with oxygen in the cells of our body to form carbon dioxide and water alongwith the production of energy.

Respiration is an exothermic process because energy is produced during this process(a) -
Whenever a solution has a pH of less than 7, it will be an acidic solution. For example, a solution having a pH of 4 will be acidic in nature (or it will be an acid). Please note that more acidic a solution is, the lower will be its pH. For example, a solution of pH 1 is much more acidic than another solution of pH 4. In other words, a solution of pH 1 will be a much more stronger acid than another acid having pH 4 (see the figure). The solutions having pH of 0, 1, 2 and 3 are usually considered to be strong acids. And the solutions having pH of 4, 5 and 6 are considered to be weak acid solutions. It is clear that the acidity of a substance is related to its pH. Strongly acidic substances have a very low pH. In fact, lower the pH, the stronger the acid.
(i) A solution turns red litmus blue. Its pH is likely to be :
(a) 1 (b) 4 (c) 5 (d) 10
(ii) The pH values of six solutions A to F are given below :
A = 0, B = 11, C = 6, D = 3, E = 13, F = 8
Which of the above solutions are acids
(a) A, C, D (b) A, B, C (c) A, C, D, F (d) A, C, D, E
(iii) Fresh milk has a pH of 6. When milk changes into curd, the pH value will :
(a) become 7 (b) become less than 6
(c) become more than 7 (d) remain unchanged
(iv) The pH values of three acids A, B and C having equal molar concentrations are 5.0, 2.8 and 3.5 respectively.
Arrange these acids in order of the increasing acid strengths.
(a) A, C, B (b) B, C, A (c) A, B, C (d) C, B, A
"Lesser the pH, stronger the acid.“
Considering the above statement, the order of increasing acidic strength is A < C < B
(v) A beaker of concentrated hydrochloric acid has a pH of 1. What colour will full range universal indicator turn if it is added to this beaker?
(a) red (b) blue (c) pink (d) no change in colour(a) -
A group of students measured the pH of some substances they found in their homes. Their results are given in the following table :
Substance pH Substance pH Apples 3.0 Vinegar 3.0 Salt 7.0 Lemon juice 2.5 Baking soda 8.5 Washing soda 11.5 Sugar 7.0 Milk 6.5 Black coffee 5.0 Household ammonia 12.0 Toothpaste 9.0 (i) Which solution is the most acidic?
(a) Apples (b) Vinegar (c) Lemon Juice (d) Black Coffee
(ii) Which solution is the most alkaline?
(a) Household ammonia (b) Washing soda
(c) Baking soda (d) Toothpaste
(iii) Which solutions are neutral?
(a) Salt (b) Sugar
(c) Milk (d) Both (i) and (ii)
(iv) What will be the litmus test if the solution is basic?
(a) Red litmus will turn to blue
(b) Blue litmus will turn to red
(c) No change in colour
(d) It will change into orange pink.
(v) Arrange the following in order of the increasing basic strengths.
Baking soda, Toothpaste, Household ammonia, Washing soda
(a) Baking soda, Toothpaste, Household ammonia, Washing soda
(b) Baking soda, Toothpaste, Washing soda, Household ammonia
(c) Washing soda, Baking soda, Toothpaste, Household ammonia
(d) Baking soda, Household ammonia, Toothpaste, Washing soda
Higher the pH, the stronger the base(a)
Case Study
*****************************************
Answers
Acids, Bases and Salts Case Study Questions With Answer Key Answer Keys
-
(i) (c): As the pH value increases from 7 to 14, it represents decrease in H+ ion concentration in the solution.
(ii) (c): pH = -logl0 [H+] = 8
logl0 [H+] =-8
[H+] = 10- 8 mol/L
(iii) (a)
(iv) (b): C2H5OH is not an ionic compound, it is a covalent compound and hence does not give H+ ions in aqueous solution.
(v) (c): (a) Lower the pH of the solution, more acidic is the solution and higher is the [H+] ions
Thus, solution P (pH = 1) has higher [H+] ions than solution R (pH = 3).
(b) Higher the pH of the solution, more basic is the solution and higher is the [OH-] ions
Thus, solution Q (pH = 9) has lower [OH-] ions than solution S (pH = l3).
(c) Solution P (pH = 1) is acidic which turns blue litmus solution red whereas solution Q (pH = 9) is basic which turns red litmus solution blue.
(d) Solution P (pH = 1) is highly acidic while solution S (pH = l3) is highly basic and solution Q (pH = 9) is weakly basic. -
(i) (a): The compound of sodium that is a constituent of baking powder and is used in antacids, is sodium hydrogen carbonate (NaHC03).
\(\text { (iii) }(\mathrm{a}): \mathrm{Na}_{2} \mathrm{CO}_{3}+2 \mathrm{H}_{2} \mathrm{O} \ \ \longrightarrow 2 \mathrm{NaOH}+ \mathrm{H}_{2} \mathrm{CO}_{3}\)
Strong base (Z)Weak acid
NaOH ionises completely to give a large amount of OH- ions whereas H2CO3 ionises partially to give a small amount ofH+ ions. Hence, the solution is overall alkaline.
(iv) (b): Z is carbonic acid, a weak acid formed when Na2CO3 is dissolved in water.
(v) (d) -
(i) (c): NaCI is insoluble in alcohol and it is a white crystalline solid. Pure NaCI is not hygroscopic in nature
(ii) (d): Aqueous solution of common salt is neutral in nature.
\(\mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O} \ \ \ \longrightarrow \mathrm{NaOH} \ + \ \ \ \ \ \mathrm{HCl}\)
Strong base Strong acid
(iv) (a): When Na2CO3 (sodium carbonate) is dissolved in water then it forms alkaline aqueous solution due to the formation of NaOH which is a strong alkali.
(v) (d): Sodium hydroxide (NaOH) is prepared by chlor-alkali process. -
(i) (d): Gypsum is CaSO4 ·2H2Oand plaster of Paris is CaSO4\(\frac{1}{2}\) H2O. Difference in number of water molecules \(=\frac{3}{2}\).
(ii) (c): Plaster of Paris is hardened by combining with water.
(iii) (c): Dead burnt plaster is CaSO4 (anhydrous calcium sulphate).
(iv) (d): Gypsum: CaSO4 ·2H2O Plaster of paris: CaSO4 ·1/2H2O
(v) (d): Gypsum on heating upto lOO°C gives plaster of Paris.
\(\begin{array}{c} \mathrm{CaSO}_{4} \cdot 2 \mathrm{H}_{2} \mathrm{O} \stackrel{100^{\circ} \mathrm{C}}{\longrightarrow} \mathrm{CaSO}_{4} \cdot \frac{1}{2} \mathrm{H}_{2} \mathrm{O}+1 \frac{1}{2} \mathrm{H}_{2} \mathrm{O} { } \end{array}\)
Gypsum Plaster of Paris
-
\(\text { (i) }(\mathrm{c}): \mathrm{CuO}+2 \mathrm{HCl} \longrightarrow \mathrm{CuCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}\)
(Bluish green)
(ii) (a): On diluting, H+ ion concentration reduces per unit volume thus, pH increases. On the other hand, on diluting, OH- concentration also reduces, pOH increases and pH decreases. As, pOH + pH = 14. Thus, pH of Q (basic solution) decreases while that of P (acidic solution) increases on dilution
(iii) (c): Formic acid is the common name of methanoic acid, and it is present in bee sting
(iv) (c)
(v) (b): Soil Y is acidic. Hence, it should be treated with powdered chalk to reduce its acidity -
\({ (i) }(\mathrm{b}): \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2} \longrightarrow 2 \mathrm{NaHCO}_{3}\)
(ii) (b): \(\mathrm{NaHCO}_{3}+\mathrm{CH}_{3} \mathrm{COOH} \longrightarrow \mathrm{CH}_{3} \mathrm{COONa}\) \(+\mathrm{CO}_{2} \uparrow+\mathrm{H}_{2} \mathrm{O}\)
Carbon dioxide gas is evolved which turns limewater milky. It extinguishes a burning splinter since it is not a supporter of combustion. It dissolves in sodium hydroxide solution and it is an odourless gas.
\({ (iii) }(\mathrm{c}): 2 \mathrm{NaHCO}_{3} \stackrel{\text { Heat }}{\longrightarrow} \mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{H}_{2} \mathrm{O}+\mathrm{CO}_{2}\)
NaHCO3 is soluble in water.
\({ (iv) }(\mathbf{b}): \mathrm{NaHCO}_{3}+\mathrm{CH}_{3} \mathrm{COOH} \longrightarrow\) \(\mathrm{CH}_{3} \mathrm{COONa}+\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}\)
(v) (c): It is not used in manufacture of soap. -
(i) (d)
(ii) (d)
(iii) (b): Bleaching powder gives chlorine on exposure to air by reacting with CO2 . \(\mathrm{CaOCl}_{2}+\mathrm{CO}_{2} \longrightarrow \mathrm{CaCO}_{3}+\mathrm{Cl}_{2}\)
(iv) (d)
\({ (v) }(\mathrm{a}): \mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{Cl}_{2} \longrightarrow \mathrm{CaOCl}_{2}+\mathrm{H}_{2} \mathrm{O}\) -
(i) (d)
(ii) (c): Na2CO3 reacts with dilute acids to give COgas with brisk effervescence.
\(\mathrm{Na}_{2} \mathrm{CO}_{3(s)}+2 \mathrm{HCl}_{(a q)} \longrightarrow 2 \mathrm{NaCl}_{(a q)}+\mathrm{H}_{2} \mathrm{O}_{(l)}\)\(+\mathrm{CO}_{2(g)} \uparrow\)
Sodium Dil. Hydrochloric Sodium Water Carbon dioxide
carbonate acid chloride
(iii) (a): Chief raw materials for the manufacture of washing soda are sodium chloride (NaCl), ammonia (NH3 ) and limestone (CaCO3)
(iv) (a): Sodium carbonate turns red litmus blue
(v) (b): Sodium hydroxide and calcium carbonate are formed when the solution of sodium carbonate and slaked lime, Ca(OH)2 is heated \(\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{Ca}(\mathrm{OH})_{2} \longrightarrow 2 \mathrm{NaOH}+\mathrm{CaCO}_{3}\) -
(i) (b): Baking soda (NaHCO3) is basic in nature.
(ii) (d): The solution turns blue litmus red, hence it is acidic.
(iii) (b): Acids turn blue litmus red, liberate hydrogen gas with zinc and evolve carbon dioxide gas with metal carbonates. Bases turn red litmus blue, evolve hydrogen gas with zinc and do not react with metal carbonates
(iv) (b):Indicator Colour in acidic Colour in basic medium Flowers of hydrangea plant (blue) Blue Blue (v) (c): Vanilla essence is an olfactory indicator. So, its smell is different in acidic and basic medium which can be detected easily by a visually impaired student.
-
(i) (c)
(ii) (a)
(iii) (b): Citric and tartaric acid are from organic substances such as lemon and tamarind respectively and they are edible. Hydrochloric acid though formed inside stomach is not edible. Carbonic acid is a mild acid and is edible in the form of soda water.
(iv) (b)
(v) (b) -
(i) (b) The powder would react to moisture and turn into solid mass
(ii) (c) Plaster of Paris
(iii) (a) 373K
(iv) (d) Crystallisation
Crystallization is defined as a process by which a chemical is converted from a liquid solution into a solid crystalline state.
(v) (d) Reactant is calcium sulphate hemihydrate, product is Gypsum -
(i) (c) Calcium hydroxide, Ca(OH)2
(ii) (b) Calcium hydroxide is basic in nature
(iii) (c) change in temperature of mixture
(iv) (b) II and III
(v) \(\text { (d) } \mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}+6 \mathrm{O}_{2} \rightarrow 6 \mathrm{CO}_{2}+6 \mathrm{H}_{2} \mathrm{O}\) -
(i) (d) 10
(ii) (a) A, C, D
(iii) (b) become less than 6
(iv) (a) A, C, B
(v) (a) red -
(i) (c) Lemon Juice
(ii) (a) Household ammonia
(iii) (d) Both (i) and (ii)
(iv) (a) Red litmus will turn to blue
(v) (b) Baking soda, Toothpaste, Washing soda, Household ammonia
Case Study


























