- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

10th Standard Science English Medium Book back Important 7 Marks Questions Question Bank Software May-12 , 2020
10th Standard Science English Medium Book back Important 7 Marks Questions
Book back Important 7 Marks Questions
10th Standard
-
Reg.No. :
Science
Time :
02:30:00 Hrs
Total Marks :
238
-
State and prove the law of conservation of linear momentum.
-
Describe rocket propulsion.
-
An object is placed at a distance 20cm from a convex lens of focal length 10cm. Find the image distance and nature of the image.
-
Explain the construction and working of a 'Compound Microscope'.
-
Derive the ideal gas equation.
-
a) State Joule’s law of heating.
b) An alloy of nickel and chromium is used as the heating element. Why?
c) How does a fuse wire protect electrical appliances? -
Explain about domestic electric circuits. (circuit diagram not required)
-
What is an echo?
a) State two conditions necessary for hearing an echo.
b) What are the medical applications of echo?
c) How can you calculate the speed of sound using echo? -
Suppose that a sound wave and a light wave have the same frequency, then which one has a longer wavelength?
a) Sound
b) Light
c) both a and b
d) data not sufficient. -
What is a nuclear reactor? Explain its essential parts with their functions.
-
Mass number of a radioactive element is 232 and its atomic number is 90. When this element undergoes certain nuclear reactions, it transforms into an isotope of lead with a mass number 208 and an atomic number 82. Determine the number of alpha and beta decay that can occur.
-
1. Calcium carbonate is decomposed on heating in the following reaction
CaCO3 → CaO + CO2
i. How many moles of Calcium carbonate are involved in this reaction?
ii. Calculate the gram molecular mass of calcium carbonate involved in this reaction.
iii. How many moles of CO2 are there in this equation? -
a) Identify the bond between H and F in HF molecule.
b) What property forms the basis of identification?
c) How does the property vary in periods and in groups? -
Vinu dissolves 50 g of sugar in 250 ml of hot water, Sarath dissolves 50 g of same sugar in 250 ml of cold water. Who will get faster dissolution of sugar? and Why?
-
A solid compound ‘A’ decomposes on heating into ‘B’ and a gas ‘C’. On passing the gas ‘C’ through water, it becomes acidic. Identify A, B and C.
-
The molecular formula of an alcohol is C4H10O. The locant number of its –OH group is 2.
(i) Draw its structural formula.
(ii) Give its IUPAC name.
(iii) Is it saturated or unsaturated? -
Where do the light dependent reaction and the Calvin cycle occur in the chloroplast.
-
Shylesh has some pet animals at his home. He has few rabbits too, one day while feeding them he observed something different with the teeth. He asked his grandfather, why is it so? What would have been the explanation of his grandfather?
-
Why are leucocytes classified as granulocytes and agranulocytes? Name each cell and mention its functions.
-
Transpiration is a necessary evil in plants. Explain.
-
Illustrate the structure and functions of brain.
-
Classify neurons based on its structure.
-
Write the physiological effects of gibberellins.
-
Susan’s father feels very tired and frequently urinates. After clinical diagnosis he was advised to take an injection daily to maintain his blood glucose level. What would be the possible cause for this? Suggest preventive measures.
-
What are the phases of menstrual cycle? Indicate the changes in the ovary and uterus.
-
Explain with an example the inheritance of dihybrid cross. How is it different from monohybrid cross?
-
The sex of the new born child is a matter of chance and neither of the parents may be considered responsible for it. What would be the possible fusion of gametes to determine the sex of the child?
-
Imprints of fossils tell us about evolution-How?
-
Octopus, cockroach and frog all have eyes. Can we group these animals together to establish a common evolutionary origin. Justify your answer.
-
Discuss the importance of biotechnology in the field of medicine.
-
Polyploids are characterised by gigantism. Justify your answer.
-
Changes in lifestyle is a risk factor for occurrence of cardiovascular diseases. Can it be modified? If yes, suggest measures for prevention.
-
What are the sources of solid wastes? How are solid wastes managed?
-
What are the consequences of soil erosion?
-
A door is pushed, at a point whose distance from the hinges is 90 cm, with a force of 40 N. Calculate the moment of the force about the hinges.
-
For a person with hypermetropia, the near point has moved to 1.5m. Calculate the focal length of the correction lens in order to make his eyes normal.
-
Keeping the temperature as constant, a gas is compressed four times of its initial pressure. The volume of gas in the container changing from 20cc (V1 cc) to V2 cc. Find the final volume V2.
-
In the circuit diagram given below, three resistors R1, R2 and R3 of 5 Ω, 10 Ω and 20 Ω respectively are connected as shown. Calculate:
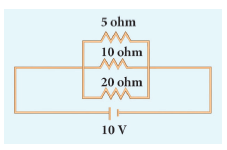
A) Current through each resistor
B) Total current in the circuit
C) Total resistance in the circuit -
A source producing a sound of frequency 90 Hz is approaching a stationary listener with a speed equal to (1/10) of the speed of sound. What will be the frequency heard by the listener?
-
Identify A, B, C, and D from the following nuclear reactions.
(i) 13AI27 + A \(\longrightarrow \) 15P30 + B
(ii) 12Mg24 + B \(\longrightarrow \) 11Na24 + C
(iii) 92U238 + B \(\longrightarrow \) 93Np239 + D -
Calculation of mass from mole
Calculate the mass of the following
i) 0.3 mole of aluminium (Atomic mass of Al = 27)
ii) 2.24 litre of SO2 gas at S.T.P
iii) 1.51 × 1023 molecules of water
iv) 5 × 1023 molecules of glucose? -
1.5 g of solute is dissolved in 15 g of water to form a saturated solution at 298K. Find out the solubility of the solute at the temperature.
-
What is the mass of sodium chloride that would be needed to form a saturated solution in 50 g of water at 30°C. Solubility of sodium chloride is 36 g at 30°C?
-
Calculate the pH of 1 × 10–4 molar solution of NaOH.
-
Calculate the pH of a solution in which the concentration of the hydrogen ions is 1.0 × 10–8 mol litre–1
Part A
34 x 7 = 238
11 x 7 = 77






 10th Standard Science Syllabus
10th Standard Science Syllabus  10th Standard Science Study Materials
10th Standard Science Study Materials

Reviews & Comments about 10th Standard Science English Medium Book back Important 7 Marks Questions
Write your Comment