- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard English Medium Chemistry Subject Book Back 1 Mark Questions with Solution Part - II Question Bank Software Jun-08 , 2021
QB365 provides detailed and simple solution for every Book back Questions in class 11 Chemistry Subject. It will helps to get more idea about question pattern in every book back questions with solution.
11th Standard English Medium Chemistry Subject Book Back 1 Mark Questions with Solution Part - II
11th Standard
-
Reg.No. :
Chemistry
Time :
00:30:00 Hrs
Total Marks :
15
-
Which one of the following represents 180 g of water ?
(a)5 Moles of water
(b)90 moles of water
(c)\(\frac { 6.022\times { 10 }^{ 23 } }{ 180 } \) molecules of water
(d)6.022\(\times\)1024molecules of water
-
If n = 6, the correct sequence for filling of electrons will be __________
(a)ns \(\rightarrow\) (n-2)f \(\rightarrow\) (n - 1)d \(\rightarrow\) np
(b)ns \(\rightarrow\) (n - 1) d \(\rightarrow\) (n - 2) f \(\rightarrow\) np
(c)ns \(\rightarrow\) (n-2)f \(\rightarrow\)np \(\rightarrow\) (n-1)d
(d)none of these are correct
-
The electronic configuration of the atom having maximum difference in first and second ionisation energies is _________
(a)1s2, 2s2, 2p6, 3s1
(b)1s2, 2s2, 2p6, 3s2
(c)1s2, 2s2, 2p6, 3s2, 3s2, 3p6, 4s1
(d)1s2, 2s2, 2p6, 3s2, 3p1
-
In case of alkali metal halides, the ionic character increases in the order ____________
(a)MF < MCI < MBr < MI
(b)MI < MBr < MCI < MF
(c)MI < MBr
(d)none of these
-
The value of universal gas constant depends upon __________
(a)Temperature of the gas
(b)Volume of the gas
(c)Number of moles of the gas
(d)units of Pressure and volume.
-
Change in internal energy, when 4 kJ of work is done on the system and 1 kJ of heat is given out by the system is ______________
(a)+1 kJ
(b)- 5 kJ
(c)+3 kJ
(d)- 3 kJ
-
An equilibrium constant of 3.2\(\times\)10–6 for a reaction means, the equilibrium is _____________
(a)largely towards forward direction
(b)largely towards reverse direction
(c)never established
(d)none of these
-
At 100o C the vapour pressure of a solution containing 6.5g a solute in 100g water is 732mm. If Kb = 0.52, the boiling point of this solution will be __________
(a)102oC
(b)100oC
(c)101oC
(d)100.52oC
-
Pick out the incorrect statement from the following.
(a)Sp3 hybrid orbitals are equivalent and are at an angle of 109o 28' with each other
(b)dsp2 hybrid orbitals are equivalent and bond angle between any two of them is 90o
(c)All five sp3d hybrid orbitals are not equivalent out of these five sp3d hybrid orbitals, three are at an angle of 120o, remainir two are perpendicular to the plane containing the other three
(d)none of these
-
The IUPAC name of the compound \({ H }_{ 3 }C-\overset { \underset { | }{ { CH }_{ 3 } } }{ \underset { \overset { | }{ { CH }_{ 3 } } }{ C } } -CH=C{ \left( { CH }_{ 3 } \right) }_{ 2 }\) is _____________
(a)2,4,4 – Trimethylpent -2-ene
(b)2,4,4 – Trimethylpent -3-ene
(c)2,2,4 – Trimethylpent -3-ene
(d)2,2,4 – Trimethylpent -2-ene
-
Which of the group has highest +I effect ?
(a)CH3-
(b)CH3 - CH2 -
(c)(CH3)2 - CH-
(d)(CH3)3 - C-
-
In the following reaction,
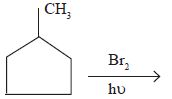
The major product obtained is ________(a) (b)
(b) (c)
(c) (d)
(d)
-
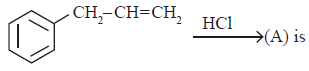 (a)
(a) (b)
(b) (c)
(c)both (a) and (b)
(d)Cl
|

-
The treatment of ethyl formate with excess of RMgX gives ____________
(a)\(R-\underset { \overset { || }{ O } }{ C } -R\)
(b)\(R-\underset { \overset { | }{ OH } }{ CH } \quad R\)
(c)R- CHO
(d)R- O- R
-
Assertion (A) : Oxygen plays a key role in the troposphere
Reason (R) : Troposphere is not responsible for all biological activities
i) Both (A) and R are correct and (R) is the correct explanation of (A)
ii) Both (A) and R are correct and (R) is not the correct explanation of (A)
iii) Both (A) and R are not correct
iv) (A) is correct but( R) is not correct
Part I
15 x 1 = 15






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard English Medium Chemistry Subject Book Back 1 Mark Questions with Solution Part - II
Write your Comment