- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard English Medium Chemistry Subject Book Back 5 Mark Questions with Solution Part - I Question Bank Software Jun-08 , 2021
QB365 provides detailed and simple solution for every Book back Questions in class 11 Chemistry Subject. It will helps to get more idea about question pattern in every book back questions with solution.
11th Standard English Medium Chemistry Subject Book Back 5 Mark Questions with Solution Part - I
11th Standard
-
Reg.No. :
Chemistry
Time :
00:30:00 Hrs
Total Marks :
55
-
A Compound on analysis gave Na = 14.31% S = 9.97% H = 6.22% and 0 = 69.5%.
Calculate the molecular formula of the compound if all the hydrogen in the compound is present in combination with oxygen as a water of crystallization. (molecular mass of the compound is 322). -
What is the de Broglie wave length of an electron, which is accelerated from the rest, through a potential difference of 100 V ?
-
What is screening effect? Briefly give the basis for pauling's scale of electronegativity.
-
A group-1 metal (A) which is present in common salt reacts with (B) to give compound (C) in which hydrogen is present in –1 oxidation state. (B) on reaction with a gas (C) to give universal solvent (D). The compound (D) on reacts with (A) to give (E), a strong base. Identify A, B, C, D and E. Explain the reactions.
-
Describe briefly the biological Importance of Calcium and magnesium.
-
Write the Van der Waals equation for a real gas. Explain the correction term for pressure and volume.
-
Calculate the enthalpy change for the reaction
Fe2O3 + 3CO ⟶ 2Fe + 3CO2 from the following data.
2Fe +\(\frac{3}{2}\)O2 ⟶ Fe2O3; ΔH = -741 kJ
C +\(\frac{1}{2}\)O2 ⟶ CO; ΔH = -137 kJ
C + O2 ⟶ CO2; ΔH = - 394.5 kJ -
1 mol of CH4, 1 mole of CS2 and 2 mol of H2S are 2 mol of H2 are mixed in a 500 ml flask. The equilibrium constant for the reaction KC = 4 x 10–2 mol2 lit–2. In which direction will the reaction proceed to reach equilibrium ?
-
Henry’s law constant for solubility of methane in benzene is 4.2 x 10-5 mm Hg at a particular constant temperature At this temperature.
Calculate the solubility of methane at
i) 750 mm Hg
ii) 840 mm Hg -
Identify the compound A, B, C and D in the following series of reactions
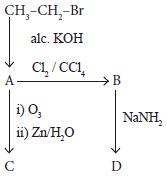
-
How is acid rain formed ? Explain its effect
Part I
11 x 5 = 55






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard English Medium Chemistry Subject Book Back 5 Mark Questions with Solution Part - I
Write your Comment