- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard English Medium Chemistry Subject Book Back 5 Mark Questions with Solution Part - II updated Book back Questions Question Bank Software May-13 , 2022
QB365 provides detailed and simple solution for every Book back Questions in class 11 Chemistry Subject. It will helps to get more idea about question pattern in every book back questions with solution.
latest Book back Questions11th Standard English Medium Chemistry Subject Book Back 5 Mark Questions with Solution Part - II
11th Standard
-
Reg.No. :
Chemistry
Time :
00:30:00 Hrs
Total Marks :
60
-
Calculate the percentage composition of the elements present in magnesium carbonate. How many kilogram of CO2 can be obtained by heating 1 kg of 90 % pure magnesium carbonate.
-
What is the de Broglie wave length of an electron, which is accelerated from the rest, through a potential difference of 100 V ?
-
Why the first ionisation enthalpy of sodium is lower than that of magnesium while its second ionisation enthalpy is higher than that of magnesium.
-
Explain preparation of hydrogen using electrolysis.
-
Describe briefly the biological Importance of Calcium and magnesium.
-
Derive the values of critical constants in terms of van der Waals constants.
-
When I-pentyne (A) is treated with 4N alcoholic KOH at 175°C, it is converted slowly into an equilibrium mixture of 1.3% I-pentyne(A) , 95.2% 2-pentyne(B) and 3.5% of 1,2 pentadiene (C) the equilibrium was maintained at 175°C, calculate ΔG0 for the following equilibria.
B \(\rightleftharpoons \)AGIO?
B \(\rightleftharpoons \)CG20? -
A sealed container was filled with 1 mol of A2 (g), 1 mol B2 (g) at 800 K and total pressure 1.00 bar. Calculate the amounts of the components in the mixture at equilibrium given that K = 1 for the reaction
A2 (g) + B2 (g) ⇌ 2AB (g) -
Henry’s law constant for solubility of methane in benzene is 4.2 x 10-5 mm Hg at a particular constant temperature At this temperature.
Calculate the solubility of methane at
i) 750 mm Hg
ii) 840 mm Hg -
Give the structure for the following compound.
3- ethyl - 2 methyl -1-pentene -
Identify the compound A, B, C and D in the following series of reactions
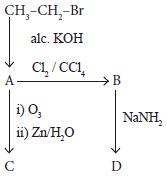
-
How is acid rain formed ? Explain its effect
Part I
12 x 5 = 60






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard English Medium Chemistry Subject Book Back 5 Mark Questions with Solution Part - II updated Book back Questions
Write your Comment