- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard English Medium Chemistry Subject Solid State Book Back 1 Mark Questions with Solution Part - II updated Book back Questions Question Bank Software May-13 , 2022
QB365 provides detailed and simple solution for every Book back Questions in class 12 Chemistry Subject. It will helps to get more idea about question pattern in every book back questions with solution.
latest Book back Questions12th Standard English Medium Chemistry Subject Solid State Book Back 1 Mark Questions with Solution Part - II
12th Standard
-
Reg.No. :
Chemistry
Time :
00:30:00 Hrs
Total Marks :
5
-
If ‘a’ is the length of the side of the cube, the distance between the body centered atom and one corner atom in the cube will be_________.
(a)\(\left( \cfrac { 2 }{ \sqrt { 3 } } \right) a\)
(b)\(\left( \cfrac { 4 }{ \sqrt { 3 } } \right) a\)
(c)\(\left( \cfrac { \sqrt { 3 } }{ 4 } \right) a\)
(d)\(\left( \cfrac { \sqrt { 3 } }{ 2 } \right) a\)
-
Potassium has a bcc structure with nearest neighbor distance 4.52 \(\overset{o}{A}\). Its atomic weight is 39. its density will be_______.
(a)915 kg m-3
(b)2142 kg m-3
(c)452 kg m-3
(d)390 kg m-3
-
Schottky defect in a crystal is observed when ______.
(a)unequal number of anions and anions are missing from the lattice
(b)Equal number of cations and anions are missing from the lattice
(c)an ion leaves its normal site and occupies an interstitial site
(d)no ion is missing from its lattice
-
Assertion: due to Frenkel defect, density of the crystalline solid decreases.
Reason: In Frenkel defect cation and anion leaves the crystal.
Codes:
a) Both assertion and reason are true and reason is the correct explanation of assertion
b) Both assertion and reason are true but reason is not the correct explanation of assertion
c) Assertion is true but reason is false
d) Both assertion and reason are false -
A two dimensional solid pattern formed by two different atoms X and Y is shown below. The black and white squares represent atoms X and Y respectively. The simplest formula for the compound based on the unit cell from the pattern is _______.
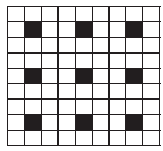 (a)
(a)XY8
(b)X4Y9
(c)XY2
(d)XY4
Part I
5 x 1 = 5






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard English Medium Chemistry Subject Solid State Book Back 1 Mark Questions with Solution Part - II updated Book back Questions
Write your Comment