- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

7th Standard Science Important Questions Question Bank Software Feb-04 , 2020
7th Standard Science Important Questions
Important Questions
7th Standard
-
Reg.No. :
Science
Time :
01:00:00 Hrs
Total Marks :
100
-
Name some of the derived quantities.
-
Give the value of one light year.
-
Write down the formula used to find the volume of a cylinder.
-
Give the formula to find the density of objects.
-
Name the liquid in which an iron ball sinks.
-
Name the units used to measure the distance between celestial objects.
-
What is the density of gold?
-
What is the area of 10 squares each having side of 1 m?
-
Find the area of the following regular shaped figures: (Take \(\pi\) = 22/7)
(a) A rectangle whose length is 12 m and breadth is 4 m.
(b) A circle whose radius is 7 m.
(c) A triangle whose base is 6 m and height is 8 m. -
Find the volume of (Take \(\pi\) = 22/7)
i. a cube whose side is 3 cm.
ii. a cylinder whose radius is 3 m and height is 7 m. -
Write the SI unit of speed
-
What is the fundamental unit of amount of substance?
-
What is the SI unit of electric charge?
-
How do you find the area of irregularly shaped figures?
-
How will you determine the volume of a liquid?
-
Which one of the following has more volume. Iron block or a wooden block of same mass.
-
Which one of the following has more density. Water or cooking oil.
-
What is the distance between the earth and proxima centauri star?
-
All objects having uniform speed need not have uniform velocity. Describe with the help of examples.
-
“She moves at a constant speed in a constant direction”. Rephrase the same sentence in fewer words using concepts related to motion.
-
Correct your friend who says “The acceleration gives the idea of how fast the position changes”.
-
Is displacement a scalar quantity?
-
Give some examples for scalar quantity
-
Mention any two conditions for stability of a body?
-
Write the chemical formula and name the elements present in the following compounds:
a. Sodium chloride
b. Potassium hydroxide
c. Carbon dioxide
d. Calcium oxide
e. Sulphur dioxide -
Classify the following molecules as the molecules of element or compound
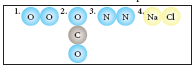
-
What do you understand by chemical formula of a compound? What is its significance?
-
Write the symbols for the following elements and classify them as solid, liquid and gas.
Aluminum, carbon, chlorine, mercury, hydrogen and helium -
Classify the following as elements and compounds.
Water, Common salt, Sugar, Carbon dioxide, Iodine and Lithium -
How many elements are known at present?
-
What is a molecule?
-
What is the atomicity of H2SO4?
-
Define - Atom.
-
Name the sub-atomic particles.
-
What is the characteristics of proton?
-
What is a neutron?
-
What is the mass of a proton?
-
What is isotone?
-
In the notation of an atom ZXA. What does A & Z represent?
-
Write two types of reproduction in plants.
-
Define – pollination.
-
What are the agents of pollination?
-
What is tendril?
-
What are thorns?
-
Describe a stamen.
-
Name the sub aerial modifications of stem.
-
Differentiate Phyllode and Phylloclade.
-
What are breathing Roots or Pneumatophores?
-
Write about the right way of protecting the eyes?
-
How to keep your hair clean and healthy?
-
Sobi frequently playing with her mobile. Suggest your ideas to protect her eye from irritation?
-
Ravi said, Ganga had minor burn, so I washed with water. Do you agree with his statement? Explain, Why?
-
List two measures to maintain community hygiene
-
Name two disease which can be prevented by vaccination.
-
What is Raster Graphics?
-
Write notes on 2D and 3D pictures.
-
What is 'Photoshop'?
-
Name the types of pictures?
-
Temperature of Srinagar (J&K) is — 40C and in Kodaikanal is 30C which of them has greater temperature? What is the difference between the temperatures of these two places?
-
Jyothi was prepared to measure the temperature of hot water with a clinical thermometer. Is it right or wrong? Why?
-
A clinical thermometer is not used to measure the temperature of air, why?
-
What is the use of kink in clinical thermometer?
-
What is the S.I unit of electrical conductivity?
-
Define fuse.
-
Name some devices that run using heat effect of electric current.
-
What is a battery?
-
State two examples of periodic changes.
-
Cold milk is heated and it becomes hot. Which type of change it is?
-
Growing of nails is a periodic change. Why?
-
What type of energy changes is associated when ice melts?
-
Which organelle uses energy from sunlight to make starch?
-
What are the main things in a nucleus?
-
What does cell membrane do?
-
Why lysosomes are known as scavengers of the cell?
-
Define – dichotomous key
-
Write two examples of Monera.
-
What is binomial nomenclature?
-
Write the binomial name of
a) Human being
b) Paddy -
What is the Shortcut key for Save option?
-
What is Tux Math?
-
What is the use of Ranger?
-
What are derived quantities?
-
Define the density of objects.
-
What is one light year?
-
Define - Astronomical unit?
-
A solid cylinder of mass 280 kg has a volume of 4 m3. Find the density of cylinder.
-
Define mass Mention its unit.
-
What is physical quantity? give example.
-
What do you mean by 'unit'?
-
What is measurement?
-
What is meant by area?
-
What is capacity of a container?
-
What is the relation between density, volume and mass?
-
Define astronomical unit
-
Define one light year.
-
Wooden block occupies more volume than the iron ball of same mass. Give reason.
-
Show the shape of the distance – time graph for the motion in the following cases.
a. A bus moving with a constant speed.
b. A car parked on a road side. -
What do you mean by constant acceleration?
-
What is centre of gravity ?
-
A body moves is a circle of radius '2R' what is the distance covered and displacement of the body after 2 complete rounds?
-
Draw distance-time graphs for the following
(i) a stationary body
(ii) a body moving with variable velocity. -
Mention the real life applications of centre of gravity.
-
Explain the concept which is used in the continuous movement of Thanjavur doll?
-
Write note on the following:
(i) Nautical mile
(ii) knot. -
Saranya jogs from one end A to the other end B of a straight road of length 320m in 2min 40 seconds. Then she turns back and jogs 100m to point C in 1 min. what is her average velocity in jogging.
-
A train starting from a railway station and moving with uniform acceleration attains a speed of 40 km/h in 10 minutes. Find its acceleration?
-
Define speed Mention its formula and unit.
-
What is non-uniform velocity? Give example
-
What is stability? Mention its types.
-
Write the chemical formula for the following elements
a. Hydrogen
b. Nitrogen
c. Ozone
d. Sulphur -
What are compounds? Give two examples.
-
Give an example for the elements derived from their Latin names
-
What is atomicity of elements?
-
Calculate the atomicity of H2SO4.
-
Aakash noticed that the metal latch on gate was difficult to open during hot sunny days. However, it was not difficult to open the same latch at night. Aakash observed that the latch and the gate are exposed to the sun during day time.
a. Formulate a hypothesis based on the information provided.
b. Briefly state how you would test the hypothesis. -
What changes take place in the movement and arrangement of particles during heating process?
-
In the diagram below, the circle, square and triangle represent the atoms of different elements.
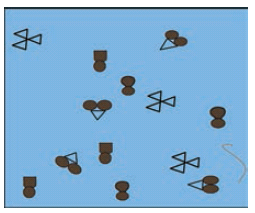
In the diagram above, identify all combinations that represent
a. A molecule of a compound
b. A molecule of an element consisting of two atoms
c. A molecule of an element consisting of three atoms -
What is an atom?
-
What are the 3 most abundant atoms on the earth?
-
Give an account of elements known to us
-
What are metalloids? Give examples
-
Give an example of a metal which
(a) is liquid at room temperature.
(b) is the best conductor of heat. -
Write any two properties of a compound.
-
What happens to matter during heating?
-
How do hot air balloons float?
-
Why is hydrogen considered as an element?
-
Write the difference between a mixture and compund.
-
Distinguish Isotopes from Isobar.
-
Differentiate mass number from atomic number.
-
An atom of an element has no electron, will that atom have any mass or not? Can an atom exist without electron? If so then give example.
-
Is the structure of the atom the same as the structure of the solar system
-
Draw the atomic structure of Oxygen
-
What is atomic mass number. Mention its formula?
-
Define isotopes? Give example.
-
Define Isobars? Give example
-
What are the limitations of Thomson's model of an atom?
-
From the symbol 15P31, state.
(i) Mass number of phosphorus.
(ii) Atomic number of phosphorus. -
What makes atoms stick together?
-
Write notes on phyllode.
-
Ginger is considered to be a stem, not a root. Why?
-
Write a note an roots modified for mechanical support.
-
How do Epiphytic roots differ from Sucking roots?
-
Write a note on phylloclade.
-
What are traps or Insectivorous plants?
-
Differentiate self pollination and cross pollination (OR) Write the differences between self and cross pollination.
-
Identify the plant and the modification seen in it.
-
Parasitic plants lack leaves
-
Why do all plants lack breathing roots?
-
Why first aid is essential?
-
A person is sleeping during day time. Why does this happen with some people that they feel sleepy during day time in office or in the classroom. Have you ever come across such situation? Explain.

-
Write a note on non-communicable diseases.
-
Write a note on Chicken pox.
-
We should use a handkarchief when we sneeze, Justify.
-
What can we do to maintain personal hygiene?
-
Richa had a pet dog. She vaccinated him regularly. He suddenly bit a stranger. Do you think that the person could have got Rabies.
-
Differentiate between Raster and Vector images.
-
Why do we use Mercury in thermometers? Can water be used instead of mercury? What are the problems in using it?
-
Swathi kept a laboratory thermometer in hot water for some time and took it out to read the temperature. Ramani said it was a wrong way of measuring temperature. Do you agree with Ramani? Explain your answer.
-
Define an electric current.
-
Differentiate parallel and serial circuits.
-
Distinguish physical and chemical changes.
-
How can a change occur in a substance?
-
What is the difference between dissolution of sugar and burning of sugar?
-
Distinguish between the following pairs Smooth ER and Rough ER Cell wall and cell membrane Chloroplast and mitochondria
-
Write correct sequence from cell to organism?
-
Write the levels of classification.
-
Write any two merits of Five Kingdom classification.
-
Describe the graphical method to find the area of an irregularly shaped plane figure.
-
How will you determine the density of a stone using a measuring jar?
-
How will you find the volume of an irregularly shaped object (stone) by using measuring cylinder?
-
How will you find the area of irregular objects?
-
Explain the types of stability with suitable examples.
-
Write about the experiment to find the centre of gravity of the irregularly shaped plate.
-
Write the differences between distance and displacement
-
Draw velocity-time graph for the following data:
1. Bus at restTime / s 0 1 2 3 4 5 Speed / ms-1 0 0 0 0 0 0 2. Bus travelling at uniform speed of m/s
Time / s 0 1 2 3 4 5 Speed / ms-1 10 10 10 10 10 10 3. Bus travelling at uniform acceleration
Time / s 0 1 2 3 4 5 Speed / ms-1 10 10 20 30 40 50 4. Bus travelling at uniform deceleration
Time / s 0 1 2 3 4 5 Speed / ms-1 50 40 30 20 10 0 5. Bus travelling with increasing acceleration
(non - uniform acceleration)Time / s 0 1 2 3 4 5 Speed / ms-1 0 2 8 18 32 50 -
The motion of a car is given in the following data draw distance - time graph.
(a) Car at restTime / s 0 1 2 3 4 5 Speed / ms 0 20 20 20 20 20 (b) Car travelling at uniform speed of 10 ms-1
Time / s 0 1 2 3 4 5 Speed / ms 0 10 20 30 40 50 (c) Car travelling at increasing speed
Time / s 0 1 2 3 4 5 Speed / ms 0 5 20 45 80 125 (d) Car travelling at decreasing speed
Time / s 0 1 2 3 4 5 Speed / ms 0 45 80 105 120 125 -
Differentiate metals and non metals.
-
Explain the characteristics of compounds
-
Differentiate between elements and compounds
-
Write any five characteristics of compounds.
-
Write down the properties of metalloids
-
(i) Draw the symbol for some elements as proposed by Dalton.
(ii) What are rules to be followed while assigning symbol to element. -
Draw the structure of an atom and explain the position of the sub-atomic particles.
-
The atomic number and the mass number of an element is 26 and 56 respectively. Calculate the number of electrons, protons and neutrons in its atom. Draw the structure.
-
What are nucleons? Why are they called so? Write the properties of the nucleons.
-
Define valency? What is the valency of the element with atomic number 8? What is the compound format by this element with hydrogen?
-
What was the model of an atom proposed by Thomson?
-
What are the conclusions/observations made by Rutherford?
-
Explain the following terms.
(i) Proton,
(ii) Electron,
(iii) Neutron. -
Explain the postulates of Dalton's atomic theory.
-
Write a brief account on pollination.
-
Explain the underground stems.
-
Write a note an sub aerial modifications of the stem.
-
Draw a diagram to show the life cycle of a plant. (OR) Draw the life cycle of plant.
-
Write about any three Communicable diseases in details.
-
List the situations in which first aid is given. What would you do if a person suffers from skin burns?
-
How the disease are transmitted from one person to the other person?
-
Write a note on causes and symptoms of Anaemia.
-
List the diseases affecting the eye, their causative agents, impact and Remedial measures.
-
With the help of Microsoft Photostory how will you create a video?
-
Draw the diagram of a clinical thermometer and label its parts.
-
Go to a veterinary doctor (a doctor who treats animals). Discuss and find out the normal temperature of domestic animals and birds.
-
Explain the construction and working of a dry cell.
-
A student made a circuit by using an electric cell, a switch, a torch bulb (fitted in the bulb holder) and copper connecting wires. When he turned on the switch, the torch bulb did not glow at all. The student checked the circuit and found that all the wire connections were tight.
What could be the possible reason for the torch bulb not glowing even when the circuit appears to be complete? -
Peeled and unpeeled banana does not look the same. Does that mean peeling banana is a chemical change?
-
A very hot glass on putting in cold water cracks. What does this change indicate?
-
Boiling of water is a physical change; but boiling of egg is a chemical change. Why?
-
Write about any three organelles in detail.
-
In a situation, how to explain, while your friend ask what is this, never seen before?
-
Give an account on the classification of invertebrates with few general features and examples.
-
Which kingdom has saprophytic, parasitic and symbiotic nutrition. Why?
2 Marks
81 x 2 = 162
3 Marks
87 x 3 = 261
5 Marks
44 x 5 = 220






 7th Standard Science Syllabus
7th Standard Science Syllabus 

Reviews & Comments about 7th Standard Science Important Questions
Write your Comment