- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

9th Standard Science Important Questions Question Bank Software Feb-04 , 2020
9th Standard Science Important Questions
-
Rulers, measuring tapes and metre scales are used to measure
(a)Mass
(b)Weight
(c)Time
(d)Length
-
The diameters of spherical objects are measured with a_________scale.
(a)pitch
(b)meter
(c)head
(d)vernier
-
Which of the following graph represents uniform motion of a moving particle?
(a) (b)
(b) (c)
(c) (d)
(d)
-
The hands of the clock, the spokes of wheel are example of _______
(a)linear motion
(b)circular motion
(c)oscillatory motion
(d)revolutionary motion
-
The focal length of a concave mirror is 5cm. Its radius of curvature is
(a)5 cm
(b)10 cm
(c)2.5 cm
-
A virtual and equal sized image is formed by ___________________mirrors.
(a)convex
(b)plane
(c)convex
(d)spherical
-
To form a real image -________________mirror is required.
(a)parallel
(b)plane
(c)convex
(d)concave
-
Filtration method is effective in separating _______ mixture
(a)Solid-solid
(b)solid-liquid
(c)liquid-liquid
(d)liquid-gas
-
Light, sound, heat, etc., are not matter. They are different forms of
(a)solids
(b)liquids
(c)gases
(d)energy
-
Energy is neither given out nor absorbed in the preparation of_____
(a)element
(b)compound
(c)mixture
(d)solvent
-
To separate two or more miscible liquids which do not differ much in the boiling points_______is employed
(a)distillation
(b)filtration
(c)decantation
(d)fractional distillation
-
Law of multiple proportions was proposed by _________
(a)J. Ritcher
(b)Rutherford
(c)John Dalton
(d)J.J Thomson
-
The circular orbits are numbered as 1,2,3,4, .... These numbers are referred as _________
(a)Principal Quantum Number
(b)Azimuthal Quantum Number
(c)Magnetic Quantum Number
(d)Spin Quantum Number
-
The outermost shell of an atom is called its________ shell.
(a)inner
(b)outer
(c)valence
(d)sub shell
-
Transpiration takes place through _____________.
(a)fruit
(b)seed
(c)flower
(d)stomata
-
Rhizophora is an example for_________________.
(a)positive geotropism
(b)Positive phototropism
(c)Positive hydrotropism
(d)Negative geotropism
-
Von Helmont conducted his experiment in the year_____________.
(a)1684
(b)1468
(c)1864
(d)1648.
-
Maize plant transpire _____________ gallons of water during its life span.
(a)34
(b)44
(c)64
(d)54
-
Dysentery is caused by
(a)Entamoeba
(b)Euglena
(c)Plasmodium
(d)Paramecium
-
The smallest bat lives in_________.
(a)America
(b)Thailand
(c)Africa
(d)Canada
-
Which one is the Mammal like reptile?
(a)DImetrodon
(b)Crocodile
(c)Lizard
(d)Snake
-
Phillippine goby is found in___________.
(a)marine water
(b)brackish water
(c)salt water
(d)fresh water
-
Any disease caused by the presence of excess vitamin is _________ .
(a)Night blindness
(b)Osteoporosis
(c)Vitaminosis
(d)Hyper vitaminosis
-
Vitamin E is otherwise known as _________ .
(a)Riboflavin
(b)Thiamine
(c)Tocopherol
(d)Calciferol
-
Lipases are enzymes which breaks down __________.
(a)Proteins
(b)Fats
(c)Carbohydrates
(d)Food
-
The specific heat capacity of water is
(a)4200 Jkg-1K-1
(b)420 Jg-1K-1
(c)0.42 Jg-1K-1
(d)4.2 Jkg-1K-1
-
The amount of heat required to raise the temperature through 10C is called_________
(a)thermal energy
(b)calorie
(c)heat capacity
(d)specific heat capacity
-
Sweating causes cooling because water has a_______
(a)high specific heat
(b)low specific heat
(c)high latent heat of fusion
(d)high latent heat of vaporisation
-
On a cold day, it is hard to open the lid of a tight container. But when you gently heat the neck you can easily open the lid. why?
(a)On heating Glass expands and lid contracts
(b)On heating lid expands more than the neck and thus slides easily
(c)Neck becomes slippery on heating
(d)Lid of the bottle cannot bear the heat.
-
In an electrolyte the current is due to the flow of
(a)electrons
(b)positive ions
(c)both (a) and (b)
(d)neither (a) nor (b)
-
A current of 2A passing through conductor produces 80 J of heat in 10 seconds. The resistance of the conductor is__________
(a)0.5\(\Omega \)
(b)2\(\Omega \)
(c)4\(\Omega \)
(d)20\(\Omega \)
-
Two resistances R1 and R2 are connected is parallel. Their equivalent resistance is__________
(a)R1+R2
(b)\(\cfrac { { R }_{ 1 }{ R }_{ 2 } }{ { R }_{ 1 }+{ R }_{ 2 } } \)
(c)\(\cfrac { { R }_{ 1 }+{ R }_{ 2 } }{ { R }_{ 1 }{ R }_{ 2 } } \)
(d)\(\sqrt { { R }_{ 1 }+{ R }_{ 2 } } \)
-
Assertion (A) : A bird perches on a high power line and nothing happens to the bird.
Reason (R) : The level of bird is very high from the ground.(a)If both assertion and reason are true and reason is the correct explanation of assertion.
(b)If both assertion and reason are true but reason is not the correct explanation of assertion.
(c)If assertion is true but reason is false
(d)If assertion is false but reason is true
-
The unit of magnetic flux density is
(a)weber
(b)weber/metre
(c)weber/metre2
(d)weber. metre2
-
Noble gases are placed in_____________ group in the modern periodic table.
(a)13th
(b)18th
(c)17th
(d)2nd
-
Assertion (A): Group 2 elements in the modern periodic table are called alkaline earth metals.
Reason (R): The oxides of group 2 elements produce alkaline solutions when they are dissolved in water(a)A is right R is wrong
(b)R explains A
(c)R does not explain A
(d)R is right A is wrong
-
Assertion (A): Noble gases are chemically inert in nature.
Reason (R) : Noble gases have stable electronic structures(a)Both A & R are right
(b)Both A & R are wrong
(c)A is right R is wrong
(d)A is wrong R is right
-
___________compounds are highly brittle
(a)Ionic
(b)Covalent
(c)Co-ordinate covalent
(d)Covalent
-
The bond which is formed by mutual sharing of electrons is called ________bond.
(a)ionic
(b)covalent
(c)co-ordinate covalent bond
(d)all the above
-
Statement (A) : Covalent compounds are bad conductor of electricity.
Reason (B) Covalent compounds contain charged particles (ions)(a)B explains A
(b)B does not explain A
(c)Both A & B are right
(d)Both A & B are wrong
-
The property which is characteristics of an Ionic compound is that
(a)it often exists as gas at room temperature
(b)it is hard and brittle
(c)it undergoes molecular reactions
(d)it has low melting point
-
_____&_______metals do not react with HCI or HNO3
(a)Gold & Magnesium
(b)Silver & Magnesium
(c)Gold & Silver
(d)Zinc & Silver
-
Bases ionise in water to form______ions
(a)H+
(b)H3O+
(c)OH-
(d)O2-
-
NaOH & KOH are____
(a)strong bases
(b)metal Oxides
(c)weak bases
(d)diacidic bases
-
White fibres of connective tissue are made up of
(a)elastin
(b)reticular fibres
(c)collagen
(d)myosin
-
__________is the smallest gland
(a)Pancreas
(b)Sublingual
(c)Parotid
(d)Submaxillary
-
The act of bringing swallowed food back to the mouth is called___________
(a)egestion
(b)ingestion
(c)micturition
(d)regurgitation
-
Gastric glands do not secrete__________
(a)renin
(b)pepsin
(c)lipase
(d)none of the above
-
Which one of the following is an example for wireless connections?
(a)Wi-Fi
(b)Electric wires
(c)VGA
(d)USB
-
Pen drive is _______device.
(a)Output
(b)Input
(c)Storage
(d)connecting cable
-
Instrument used to measure relative density
(a)Hydrometer
(b)Lactometer
(c)Barometer
(d)Pycnometer
-
An iron ball is weighed in air and then in water by a spring balance.
(a)Its weight in air is more than in water.
(b)Its weight in water is more than in air
(c)Its weight is same both in air and water.
(d)Its weight is zero in water.
-
If the speed of a wave is 340 m s-1 and its frequency is 1700 Hz, then wavelength λ for this wave in cm will be
(a)34
(b)20
(c)15
(d)0.2
-
Which of the following is not a planet of our solar system?
(a)Sirius
(b)Mercury
(c)Saturn
(d)Earth
-
The member of our solar system, with highly tilted orbit is _____________.
(a)Earth
(b)Pluto
(c)Mars
(d)Saturn
-
Graphene is one atom thick layer of carbon obtained from
(a)Diamond
(b)Fullerene
(c)Graphite
(d)Gas Carbon
-
1% solution of Iodoform is used as
(a)antipyretic
(b)antimalarial
(c)antiseptic
(d)antacid
-
Increased amount of ___________ in the atmosphere, results in greenhouse effect and global warming
(a)carbon monoxide
(b)sulphur dioxide
(c)nitrogen dioxide
(d)carbon dioxide
-
__________ is the method of growing plants without soil.
(a)Horticulture
(b)Hydroponics
(c)Pomology
(d)None of these.
-
Mosquito borne viral diseases are
(a)malaria and yellow fever
(b)dengue and chikungunya
(c)filariasis and typhus
(d)kala azar and diptheria
-
Convert: 104°F into Celsius scale.
-
Calculate the correct readings of the Vernier caliper L.C. 0.01 em Zero correction - Nil.
S.No. M.S.R V.C Observed Reading = M.S.R + (V.C x L.C) Correct Reading 1 3 4 2 3 7 -
Complete the table:
Power of 10 Prefix Symbol 1012 ___(i)___ T ___(ii)___ Kilo K 1015 Peta ___(iii)__ 109 ___(iv)__ ___(v)__ -
What is acceleration?
-
How is washing machine wash the clothes?
-
If an object is placed at the focus of a concave mirror, where is the image formed?
-
What is convex mirror?
-
What is principal axis?
-
Fill in the numbered blanks to make the heating curve meaningful.
-
Is air a pure substance or Mixture? Justify.
-
Arrange the following in the increasing order of atomic number
Calcium, Silicon, Boron, Magnesium, Oxygen, Helium, Neon, Sulphur, Fluorine and Sodium -
What is combination reaction ?
-
Which flowering plant shows photonasty just opposite to that of Dandelion?
-
Define taxonomy?
-
Are Reptilian eggs covered with shells?
-
Differentiate : Kwashiorkar from Marasmus
-
How many types of adulterants are there?
-
How computer generations are categorised?
-
Water is used as a coolant in car radiators. Why?
-
Does a solar cell always maintain the potential across its terminals constant? Discuss.
-
Define magnetic effect of electric current.
-
State Newlands' Law of Octaves
-
Complete the equation Na2CO3+2HCl ⟶ ?+? +CO2↑
-
What is crossing over?
-
What is parturition?
-
What is a satellite? What are the two types of satellites?
-
Who is called 'Father of Modern Organic Chemistry'?
-
What is Chemotherapy?
-
Differentiate mass and weight.
-
The mass of an object is 5 kg. What is its weight on the earth?
-
What remains constant in uniform circular motion? And What Changes continuously in uniform circular motion?
-
What is the unit of refractive index?
-
Oxygen is very essential for us to live. It forms 21% of air by volume. Is it an element or compound?
-
Distinguish between dispersed phase and dispersion medium.
-
Methane burns in oxygen to form carbon dioxide and water vapour as given by the equation
CH4(g) + 2O2(g) ➝ CO2(g)+ 2H2O(g)
Calculate: (i) the volume of oxygen needed to burn completely 50 cm3 of methane and (ii) the volume of carbon dioxide formed in this case. -
What are isotones? Give example.
-
Imagine that student A studied the importance of certain factors in photosynthesis. He took a potted plant and kept it in dark for over 24 hours. In the early hours of the next morning, he covered one of the leaves with dark paper in the centre only. Then he placed the plant in sunlight for a few hours and tested the leaf which was covered with black paper for starch.
(a) What aspect of photosynthesis was being investigated
(b) Why was the plant kept in the dark before the experiment?
(c) How will you prove that starch is present in the leaves?
(d) Name the raw materials needed for photosynthesis? -
Comment on the aquatic and terrestrial habits of amphibians.
-
Look at the picture and answer the question that follows
a) Name the process involved in the given picture.
b) Which diary food is preserved by this process?
c) What is the temperature required for the above process?
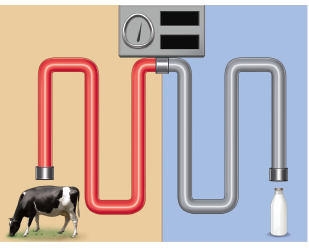
-
What is Pasterurization?
-
What is data processing?
-
Can two magnetic lines of force intersect? Justify your answer.
-
What are the limitations of Mendeleev's periodic table?
-
Explain Octet rule with an example.
-
Ionic compounds are crystalline solids at room temperature.
-
What are organic acids? Given examples?
-
What is complex tissue? Name the various kinds of complex tissues.
-
Reproductive organs are also considered as endocrine glands
-
What are the types of monitor?
-
What is meant by atmospheric pressure?
-
Write any two practical application of sound waves.
-
What are Biometrics?
-
According to you, which process of water cycle is adversely affected by human activities?
-
What is the nutritional importance of fish liver oils? Name any two marine fishes which yield these oils.
-
Sanjay had an attack of chicken pox and has just recovered. The health officer of his locality says that the disease would not occur again for him. What would be the reason for this?
-
Explain the method to find the diameter of the sphere.
-
A racing car has a uniform acceleration of 4 ms-2. What distance it covers in 10 s after start?
-
The ray of light enters from air to Kerosene? Refractive index of Kerosene is 1.41. Calculate the velocity of light in Kerosene.
-
Write the properties of mixture.
-
Lead forms three oxides A, B and C. The quantity of oxygen in each of the oxides A, B and C is 7.143%, 10.345% and 13.133% respectively. Show that the law of multiple proportions is obeyed.
-
Design an experiment to demonstrate hydrotropism.
-
Draw the life cycle of jelly fish.
-
Write a brief note on mineral nutrients.
-
List out the generations of computer
-
Convert the following:
(1) 1000F to 0C
(2) 400C to Fahrenheit (0F)
(3) 350C to Kelvin
(4) 800K to 0C -
Calculate the effective resistance between A and B as shown is the figure
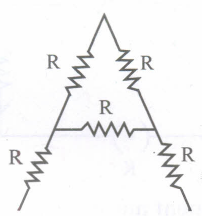
-
Explain the principle, construction and working of a AC generator.
-
Write the advantages of Modern Periodic Table.
-
List down the differences between Ionic and Covalent compounds.
-
Describe the classification of bases based on their acidity.
-
Explain the components of phloem tissue
-
Write a note on functions of liver in digestion.
-
a) When a golf ball is lowered into a measuring cylinder containing water, the water level rises by 40cm3, when the ball is completely submerged. If the mass of the ball in air is 44g. Calculate its density.
b) A 5kg sheet of tin sinks in water but if the same sheet is converted into a boat or a box, it floats. Give reason. -
Write any five applications of ultrasonic waves.
-
List out the drawbacks of Nanomaterials in chemistry.
Part - A
60 x 1 = 60
Part - B
30 x 2 = 60
Part - C
30 x 3 = 90
Part - D
20 x 5 = 100






 9th Standard Science Syllabus
9th Standard Science Syllabus  9th Standard Science Study Materials
9th Standard Science Study Materials

Reviews & Comments about 9th Standard Science Important Questions
Write your Comment