- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium - Important 1 Mark MCQ's Question Paper and Answer Key 2022 - 2023 Study Materials Dec-31 , 2022
QB365 provides a detailed and simple solution for every Possible Questions in Class 12 Chemistry Subject - Important 1 Mark MCQ's, English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
12th Standard Chemistry Important 1 Mark Questions
12th Standard
-
Reg.No. :
Chemistry
Time :
01:00:00 Hrs
Total Marks :
50
-
Which one of the following reaction represents calcinations?
(a)\(2Zn+{ O }_{ 2 }\rightarrow 2ZnO\)
(b)\(2ZnS+3O_{ 2 }\rightarrow 2ZnO+2SO_{ 2 }\)
(c)\(MgCO_{ 3 }\rightarrow MgO+CO_{ 2 }\)
(d)Both (a) and (c)
-
Zinc is obtained from ZnO by________.
(a)Carbon reduction
(b)Reduction using silver
(c)Electrochemical process
(d)Acid leaching
-
Which of the following is not true with respect to Ellingham diagram?
(a)Free energy changes follow a straight line. Deviation occurs when there is a phase change.
(b)The graph for the formation of CO2 is a straight line almost parallel to free energy axis.
(c)Negative slope of CO shows that it becomes more stable with increase in temperature.
(d)Positive slope of metal oxides shows that their stabilities decrease with increase in temperature.
-
In diborane, the number of electrons that accounts for banana bonds is ________.
(a)six
(b)two
(c)four
(d)three
-
The repeating unit in silicone is_______.
(a)SiO2
(b)(c) (d)
(d)
-
The compound that is used in nuclear reactors as protective shields and control rods is _________.
(a)Metal borides
(b)metal oxides
(c)Metal carbonates
(d)metal carbide
-
An element belongs to group 15 and 3rd period of the periodic table, its electronic configuration would be_______.
(a)1s2 2s2 2p4
(b)1s2 2s2 2p3
(c)1s2 2s2 2p6 3s2 3p2
(d)1s2 2s2 2p6 3s2 3p3
-
Among the following the correct order of acidity is ________.
(a)HClO2 < HClO < HClO3 < HClO4
(b)HClO4 < HClO2 < HClO < HClO3
(c)HClO3 < HClO4 < HClO2 < HClO
(d)HClO < HClO2 < HClO3 < HClO4
-
Among the transition metals of 3d series, the one that has highest negative \(\left( \frac { M^{ 2+ } }{ M } \right) \) standard electrode potential is _______.
(a)Ti
(b)Cu
(c)Mn
(d)Zn
-
How many moles of I2 are liberated when 1 mole of potassium dichromate react with potassium iodide?
(a)1
(b)2
(c)3
(d)4
-
Which one of the following is not correct?
(a)La(OH)3 is less basic than Lu(OH)3
(b)In lanthanoid series ionic radius of Ln3+ ions decreases
(c)La is actually an element of transition metal series rather than lanthanide series
(d)Atomic radii of Zr and Hf are same because of lanthanide contract
-
As per IUPAC guidelines, the name of the complex [Co(en)2(ONO)Cl]Cl is _______.
(a)chloro bis ethylenediamine nitrocobalt(III) chloride
(b)chloridobis(ethane-1, 2-diamine)nitro K-O Cobaltate(III) chloride
(c)chloridobis(ethane-1, 2-diammine)nitrito K-O Cobalt(II) chloride
(d)chloridobis(ethane-1, 2-diamine)nitrito K-O Cobalt(III) chloride
-
A complex in which the oxidation number of the metal is zero is_______.
(a)K4[Fe(CN)6]
(b)[Fe(CN)3(NH3)3]
(c)[Fe(CO)5]
(d)both (b) and (c)
-
In calcium fluoride, having the fluorite structure the coordination number of Ca2+ ion and F- Ion are ________.
(a)4 and 2
(b)6 and 6
(c)8 and 4
(d)4 and 8
-
If ‘a’ is the length of the side of the cube, the distance between the body centered atom and one corner atom in the cube will be_________.
(a)\(\left( \cfrac { 2 }{ \sqrt { 3 } } \right) a\)
(b)\(\left( \cfrac { 4 }{ \sqrt { 3 } } \right) a\)
(c)\(\left( \cfrac { \sqrt { 3 } }{ 4 } \right) a\)
(d)\(\left( \cfrac { \sqrt { 3 } }{ 2 } \right) a\)
-
Among the following graphs showing variation of rate constant with temperature (T) for a reaction, the one that exhibits Arrhenius behavior over the entire temperature range is _______.
(a) (b)
(b) (c)
(c) (d)
(d)both (b) and (c)
-
For the reaction, 2NH3 ⟶ N2 + 3H2, if \(\frac { -d[NH_{ 3 }] }{ dt } \) = k1[NH3], \(\frac { d[N_{ 2 }] }{ dt } =k_{ 2 }[NH_{ 3 }],\frac { d[{ H }_{ 2 }] }{ dt } \)= k3[NH3] then the relation between k1, k2 and k3 is _________.
(a)k1 = k2 = k3
(b)k1 = 3k2 = 2k3
(c)1.5k1 = 3k2 = k3
(d)2k1 = k2 = 3k3
-
In a first order reaction x ⟶ y; if k is the rate constant and the initial concentration of the reactant x is 0.1M, then, the half life is_____.
(a)\(\left( \frac { \log2 }{ k } \right) \)
(b)\(\left( \frac { 0.693 }{ (0.1)k } \right) \)
(c)\(\left( \frac { In2 }{ k } \right) \)
(d)none of these
-
The solubility of BaSO4 in water is 2.42 × 10-3gL-1 at 298K. The value of its solubility product(Ksp) will be (Given molar mass of BaSO4 =233g mol-1)
(a)1.08 × 10-14mol2L-2
(b)1.08 × 10-12mol2L-2
(c)1.08 × 10-10mol2L-2
(d)1.08 × 10-8mol2L-2
-
Which of the following relation is correct for degree of hydrolysis of ammonium acetate?
(a)\(h=\sqrt { \frac { { K }_{ h } }{ C } } \)
(b)\(h=\sqrt { \frac { { K }_{ a } }{ K_b } } \)
(c)\(h=\sqrt { \frac { { K }_{ w } }{ { K }_{ a }.{ K }_{ b } } } \)
(d)\(h=\sqrt { \frac { { { K }_{ a }.{ K }_{ b } } }{ { K }_{ w } } } \)
-
The button cell used is watches function as follows
Zn (s) + Ag2O (s) + H2O (l) ⇌ 2Ag (s) + Zn2+ (aq) + 2OH-(aq) the half cell potentials are Ag2O (s) + H2O (l) + 2e- → 2Ag (s) + 2OH- (aq) Eo = 34V and Zn (s) → Zn2+ (aq) + 2e− E0 = 0.76V . The cell potential will be_______.(a)0.84V
(b)1.34V
(c)1.10V
(d)0.42V
-
During electrolysis of molten sodium chloride, the time required to produce 0.1mole of chlorine gas using a current of 3A is _____.
(a)55 minutes
(b)107.2 minutes
(c)220 minutes
(d)330 minutes
-
Consider the change in oxidation state of Bromine corresponding to different emf values as shown in the diagram below:
\({ BrO }_{ 4 }^{ - }\overset { 1.82V }{ \longrightarrow } { BrO }_{ 3 }^{ - }\overset { 1.5V }{ \longrightarrow } HBrO\overset { 1.595v }{ \longrightarrow } { Br }_{ 2 }\overset { 1.0652V }{ \longrightarrow } Br^{ - }\)
Then the species undergoing disproportional is(a)Br2
(b)BrO4-
(c)BrO-3
(d)HBrO
-
Hair cream is _____.
(a)gel
(b)emulsion
(c)solid sol
(d)sol.
-
Collodion is a 4% solution of which one of the following compounds in alcohol – ether mixture?
(a)Nitroglycerine
(b)Cellulose acetate
(c)Glycoldinitrate
(d)Nitrocellulose
-
On which of the following properties does the coagulating power of an ion depend?
(a)Both magnitude and sign of the charge on the ion.
(b)Size of the ion alone
(c)the magnitude of the charge on the ion alone
(d)the sign of charge on the ion alone.
-
The X is _______.
(a)(b)(c)(d)None of these
-
on treatment with Con H2SO4, predominately gives______.
(a)(b)(c)(d) -
On reacting with neutral ferric chloride, phenol gives ______.
(a)red colour
(b)violet colour
(c)dark green colour
(d)no colouration.
-
Predict the product Z in the following series of reactions Ethanoic acid \(\overset { { PCI }_{ 5 } }{ \longrightarrow } X\overset { { C }_{ 6 }{ H }_{ 6 } }{ \underset { Anhydrous \ AlCI }-_{3}{ \longrightarrow } } Y\overset { 1){ CH }_{ 3 }MgBr }{ \underset { II){ H }_{ 3 }{ O }^{ + } }{ \longrightarrow } } Z.\)
(a)(CH3)2 C(OH)C6H5
(b)CH3CH(OH)C6H5
(c)CH3CH(OH)CH2- CH3
(d) -
The IUPAC name of
(a)but – 3- enoicacid
(b)but – 1- ene-4-oic acid
(c)but – 2- ene-1-oic acid
(d)but -3-ene-1-oicacid
-
In which of the following reactions new carbon – carbon bond is not formed?
(a)Aldol condensation
(b)Friedel craft reaction
(c)Kolbe’s reaction
(d)Wolf kishner reduction
-
CH3CH2 Br \(\overset { aqNaOH }{ \underset { \Delta }{ \longrightarrow } } A\overset { { KMnO }_{ 4 }{ /H }^{ + } }{ \underset { \Delta }{ \longrightarrow } } B\overset { { NH }_{ 3 } }{ \underset { \Delta }{ \longrightarrow } } C\overset { { Br }_{ 2 }/NaOH }{ \longrightarrow } D\) D' is________.
(a)bromomethane
(b)α - bromo sodium acetate
(c)methanamine
(d)acetamide
-
_______.
(a)H3PO2 and H2O
(b)H+/H2O
(c)HgSO4/H2SO4
(d)Cu2Cl2
-
Identify X in the sequence give below
(a)(b)(c)(d) -
The correct statement regarding RNA and DNA respectively is ______.
(a)the sugar component in RNA is an arabinos and the sugar component in DNA is ribose
(b)the sugar component in RNA is 2’-deoxyribose and the sugar component in DNA is arabinose
(c)the sugar component in RNA is an arabinose and the sugar component in DNA is 2’-deoxyribose
(d)the sugar component in RNA is ribose and the sugar component in DNA is 2’-deoxyribose
-
Which one of the following structures represents nylon 6,6 polymer?
(a)(b)(c)(d) -
Which is the monomer of neoprene in the following?
(a)(b)\(\mathrm{CH}_{2}=\mathrm{CH}-\mathrm{C} \equiv \mathrm{CH}\)
(c)\(\mathrm{CH}_{2}=\mathrm{CH}-\mathrm{CH}=\mathrm{CH}_{2}\)
(d) -
Among the following, one does not belong to calcination, Pick the odd one out.
(a)\({ PBCO }_{ 3 }\overset { \Delta }{ \longrightarrow } PBO+{ CO }_{ 2 }\uparrow \)
(b)\({ CaCo }_{ 3 }\overset { \Delta }{ \longrightarrow } Cao+{ CO }_{ 2 }\uparrow \)
(c)\(PbS{ O }_{ 3 }\overset { \Delta }{ \rightarrow } PbO+{ 2SO }_{ 2 }\uparrow \)
(d)\({ ZnCO }_{ 3 }\overset { \Delta }{ \rightarrow } ZnO+{ CO }_{ 2 }\uparrow \)
-
SiO44- ion has ____ geometry.
(a)Triangular
(b)Tetrahedral
(c)Linear
(d)Pentagonal bipyramidal
-
The C60 molecule is called as_________ because of its structure.
(a)buck minster fullerene
(b)bucky balls
(c)diamond
(d)both (a) & (b)
-
Pick the wrong one among the following
(a)F2 - Yellow
(b)Br2 - Red
(c)Cl2 - Colourless
(d)I2- Violet
-
The general electronic configuration of 4f series of elements can be written as ________.
(a)[Xe]4f2-14 5d0-46s2
(b)[Xe]4f2-145d0-46s1
(c)[Xe] 4f1-14506s1
(d)[Xe]4f2-145d0-46s1
-
Among the following complexes the one which shows zero crystal field stabilisation energy is _______.
(a)[Mn(H2O)6]3+
(b)[Fe(H2O)6]3+
(c)[CO(H2O)6]2+
(d)[CO(H2O)6]3+
-
Identify, the geometrical isomer of platinum complex with the ligands NH3 & Cl
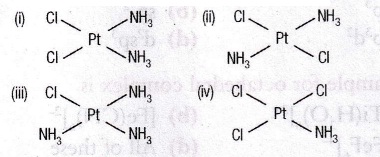
Choose the correct option(a)i & ii
(b)ii & iii
(c)i & iii
(d)ii & iv
-
The empty space between the shaded balls and hollow balls as shown in the diagram is called, _______.
(a)Hexagonal void
(b)Octahedral void
(c)Tetrahedral void
(d)Double triangular void
-
(a)
It is a 2 steps reaction, step 1 is slower than step 2
(b)It is a 2 steps reaction, step 2 is slower than step 1.
(c)Single step reaction where B is a activated complex
(d)Single step reaction in which B is a reaction intermediate.
-
Concentration is expressed in?
(a)\(\frac { number\ of\ moles/litre }{ time\ in\ sec } \)
(b)\(\frac { time\ in\ sec }{ number\ of\ moles/litre } \)
(c)\(\frac { number\ of\ moles/litre }{ volume } \)
(d)\(\frac { volume }{ number\ of\ moles/litre } \)
-
The relationship between degree of dissociation of a weak acid and its dissociation constant in a very dilute solution is _______.
(a)Ka = α2C
(b)Ka = \(\frac{α^2C}{(1+α)}\)
(c)Ka = \(\frac{α^2}{(1+α)C}\)
(d)Ka = \(\frac{α}{C(1+α)}\)
-
Using the data given below find out the strongest reducing agent ______.
\({ E }_{ { Cr }_{ 2 }{ O }_{ 7 }^{ 2- } }^{ o }{ Cr }^{ 3+ }=1.33V{ ,E }_{ { Cl }_{ 2 }{ / }{ Cl }^{ - } }^{ o }=1.36V\)
\({ E }_{ { Mn }O_{ 4 }^{ - } }^{ 0 }/{ Mn }^{ 2+ }=1.51V,{ E }_{ { Cr }^{ 3+ }/Cr }^{ o }=-0.74V\)(a)Cr
(b)Cr3+
(c)Cl-
(d)Mn2+
Answer all the following questions.
50 x 1 = 50
*****************************************
Answers
-
(c)
\(MgCO_{ 3 }\rightarrow MgO+CO_{ 2 }\)
-
(a)
Carbon reduction
-
(b)
The graph for the formation of CO2 is a straight line almost parallel to free energy axis.
-
(c)
four
-
(b)
-
(a)
Metal borides
-
(d)
1s2 2s2 2p6 3s2 3p3
-
(d)
HClO < HClO2 < HClO3 < HClO4
-
(a)
Ti
-
(c)
3
-
(a)
La(OH)3 is less basic than Lu(OH)3
-
(d)
chloridobis(ethane-1, 2-diamine)nitrito K-O Cobalt(III) chloride
-
(c)
[Fe(CO)5]
-
(c)
8 and 4
-
(d)
\(\left( \cfrac { \sqrt { 3 } }{ 2 } \right) a\)
-
(b)
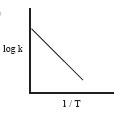
-
(c)
1.5k1 = 3k2 = k3
-
(c)
\(\left( \frac { In2 }{ k } \right) \)
-
(c)
1.08 × 10-10mol2L-2
-
(c)
\(h=\sqrt { \frac { { K }_{ w } }{ { K }_{ a }.{ K }_{ b } } } \)
-
(c)
1.10V
-
(b)
107.2 minutes
-
(d)
HBrO
-
(b)
emulsion
-
(d)
Nitrocellulose
-
(a)
Both magnitude and sign of the charge on the ion.
-
(a)
-
(b)
-
(b)
violet colour
-
(a)
(CH3)2 C(OH)C6H5
-
(a)
but – 3- enoicacid
-
(d)
Wolf kishner reduction
-
(c)
methanamine
-
(a)
H3PO2 and H2O
-
(a)
-
(d)
the sugar component in RNA is ribose and the sugar component in DNA is 2’-deoxyribose
-
(d)
-
(a)
-
(c)
\(PbS{ O }_{ 3 }\overset { \Delta }{ \rightarrow } PbO+{ 2SO }_{ 2 }\uparrow \)
-
(b)
Tetrahedral
-
(d)
both (a) & (b)
-
(c)
Cl2 - Colourless
-
(a)
[Xe]4f2-14 5d0-46s2
-
(b)
[Fe(H2O)6]3+
-
(a)
i & ii
-
(b)
Octahedral void
-
(a)
It is a 2 steps reaction, step 1 is slower than step 2
-
(a)
\(\frac { number\ of\ moles/litre }{ time\ in\ sec } \)
-
(a)
Ka = α2C
-
(a)
Cr






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium - Important 1 Mark MCQ's Question Paper and Answer Key 2022 - 2023
Write your Comment