- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium - Important 2 Mark Question Paper and Answer Key 2022 - 2023 Study Materials Dec-31 , 2022
QB365 provides a detailed and simple solution for every Possible Questions in Class 12 Chemistry Subject - Important 2 Mark English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
12th Standard Chemistry Important 2 Mark Questions
12th Standard
-
Reg.No. :
Chemistry
Time :
01:30:00 Hrs
Total Marks :
60
-
Give the uses of zinc.
-
The selection of reducing agent depends on the thermodynamic factor: Explain with an example.
-
Complete the following reactions.
a. \(B(OH)_3 + NH_3\longrightarrow \)
b. \(Na_{ 2 }B_{ 4 }{ O }_{ 7 }+{ { H }_{ 2 }{ SO }_{ 4 }+{ 5H }_{ 2 }O\longrightarrow }\)
c. \({ B }_{ 2 }{ H }_{ 6 }+2NaOH+2{ H }_{ 2 }O\longrightarrow \)
d. \({ B }_{ 2 }{ H }_{ 6 }+6{ CH }_{ 3 }OH\longrightarrow \)
e. \(4{ BF }_{ 3 }+3{ H }_{ 2 }O\longrightarrow \)
f. \(HCOOH+{ H }_{ 2 }{ SO }_{ 4 }\longrightarrow \)
g. \(2SiCl_{ 4 }+NH\)3
h. SiCl4 + 4C2H5OH \(\rightarrow\)
i. 2\(B+6NaOH\longrightarrow \)
j. \({ H }_{ 2 }{ B }_{ 4 }{ O }_{ 7 }\overset { Red\ hot }{ \rightarrow } \) -
Give the uses of argon.
-
What are interstitial compounds?
-
In an octahedral crystal field, draw the figure to show splitting of d orbitals
-
Why ionic crystals are hard and brittle?
-
Consider the oxidation of nitric oxide to form NO2
2NO(g) + O2(g) ➝2NO2(g)
(a). Express the rate of the reaction in terms of changes in the concentration of NO,O2 and NO2.
(b). At a particular instant, when [O2] is decreasing at 0.2 mol L−1s−1 at what rate is [NO2] increasing at that instant? -
Rate constant k of a reaction varies with temperature T according to the following Arrhenius equation \(\log K=\log A-\frac { { E }_{ a } }{ 2.303R } \left( \frac { 1 }{ T } \right) \)Where Ea is the activation energy. When a graph is plotted for log k Vs \(\frac{1}{T}\) a straight line with a slope of -4000K is obtained. Calculate the activation energy.
-
When aqueous ammonia is added to CuSO4 solution, the solution turns deep blue due to the formation of tetra ammine copper (II) complex,\({ [Cu({ H }_{ 2 }O)_4] }_{ (aq) }^{ 2+ }+ 4{ NH }_{ 3 }(aq)\rightleftharpoons { [Cu{ ({ NH }_{ 3 }) }_{ 4 }] }_{ (aq) }^{ 2+ }\) among H2O and NH3 Which is stronger Lewis base.
-
A solution of 0.10M of a weak electrolyte is found to be dissociated to the extent of 1.20% at 25oC. Find the dissociation constant of the acid.
-
Explain the function of H2 - O2 fuel cell.
-
The resistance of a conductivity cell is measured as 190 Ω using 0.1M KCl solution (specific conductance of 0.1M KCl is 1.3 Sm-1). When the same cell is filled with 0.003 M sodium chloride solution, the measured resistance is 6.3KΩ. Both these measurements are made at a particular temperature. Calculate the specific and molar conductance of NaCl solution.
-
What happens when a colloidal sol of Fe(OH)3 and As2S3 are mixed?
-
Comment on the statement: Colloid is not a substance but it is a state of substance.
-
Complete the following reactions
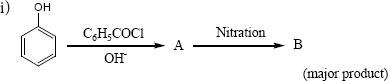
ii) \(C_6H_5-CH_{2}CH(OH)CH(CH_3)_2 \overset{ConH_2SO_4}\longrightarrow\) -
How will you convert benzaldehyde into the following compounds?
(i) benzophenone
(ii) benzoic acid
(iii) α-hydroxyphenylaceticacid. -
Complete the following reaction
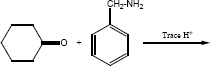
-
Give two difference between Hormones and vitamins.
-
Write the structural formula of aspirin.
-
Name some reducing agents used to convert Metal oxides to metal
-
What is burnt alum?
-
Arrange the following as indicated below:
(i) F2, Cl2, Br2, I2 - increasing bond dissociation enthalpy.
(ii) HF, HCI, HBr, HI - increasing acidic strength. -
Complete the following equations.
(i) 2 CrO42- + 2H+ ⟶
(ii) KMnO4 ⟶ -
Calculate the magnetic moment of [Fe(H2O)6]2+, if atomic number of Fe is 26.
-
Frenkel defect is not show by alkali metal halides but silver halides do. Give reason.
-
Define:- Half life period.
-
Define degree of dissociation.
-
What are secondary cells?
-
What is Tyndall effect?
Answer all the following questions.
30 x 2 = 60
*****************************************
Answers






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium - Important 2 Mark Question Paper and Answer Key 2022 - 2023
Write your Comment