- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Chemistry - Biomolecules Model Question Paper Question Bank Software Jan-03 , 2020
Biomolecules
Biomolecules Model Question Paper
12th Standard
-
Reg.No. :
Chemistry
Time :
01:00:00 Hrs
Total Marks :
40
-
Which one given below is a non-reducing sugar?
(a)Glucose
(b)Sucrose
(c)maltose
(d)Lactose
-
The pyrimidine bases present in DNA are ______.
(a)Cytosine and Adenine
(b)Cytosine and Guanine
(c)Cytosine and Thiamine
(d)Cytosine and Uracil
-
Among the following L- serine is ______.
(a)(b)(c)(d) -
Name the base present only in RNA
(a)adenine
(b)guanine
(c)uracil
(d)thymine
-
The amino acid without chiral carbon is
(a)Glycine
(b)Alanine
(c)Proline
(d)Tyrosine
-
Identify the monosaccharide among the following
(a)glucose
(b)fructose
(c)galactose
(d)all the above
-
Lactose on hydrolysis gives
(a)two molecules of glucose
(b)glucose and fructose
(c)two molecules of fructose
(d)glucose and galactose
-
The number of optical isomers are calculated by____________
(a)2n+2
(b)2n-2
(c)2n
(d)2n/2
-
Oxidation of gluconic acid with nitric acid gives__________
(a)saccharicacid
(b)CO2 and water
(c)acetic acid
(d)maltose
-
The helical structure of protein is stabilised by_________
(a)peptide bonds
(b)disulphide bonds
(c)hydrogen bonds
(d)Vanderwaals inter action
-
Which among the following is correct regarding nucleic acids?
a) Hydrolysis of DNA and RNA gives two components, a pentose sugar and phosphate group
b) Both DNA and RNA have thymine as one of 5 the pyrimidines
c) The two major purine bases of both RNA and DNA are adenine and guanine.
d) The pyrimidine base present only in DNA is uracil. -
Assertion: Vitamin D can be stored in our body.
Reason: Vitamin D is a fat soluble vitamin
Codes:
a) (A) and (R) are true and (R) is the correct explanation of (A)
b) Both (A) and (R) are true but (R) does not explain (A)
c) (A) is true but (R) is false
d) Both (A) and (R) are false -
Select the incorrect statement among the following
a) Hemoglobin is soluble in water
b) α - Kelatin is soluble in water
c) Cellulose is a polymer of glucose
d) Chlorophyll is responsible for the synthesis of carbohydrates in plants -
Identify whether the following compounds are D or L isomers.
-
Why is glucose known as dextrose?
-
The sucrose that we eat in daily life is converted into glucose and fructose. Name the enzyme which facilitates this chemical reaction.
-
How are vitamins classified?
-
Write a reaction that indicates the presence of an aldehyde group in glucose.
-
What is starch? What are the ultimate hydrolysis products?
-
Is the following sugar, D – sugar or L – sugar?
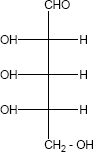
-
Show the formation of a peptide bond with an equation.
10 x 1 = 10
1 x 1 = 1
1 x 2 = 2
1 x 2 = 2
3 x 2 = 6
3 x 3 = 9
2 x 5 = 10






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Chemistry - Biomolecules Model Question Paper
Write your Comment