- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students
Full Portion Five Marks Questions
12th Standard
-
Reg.No. :
Chemistry
Time :
02:00:00 Hrs
Total Marks :
100
-
Explain the principle of electrolytic refining with an example.
-
Write a note on zeolites.
-
How will you convert boric acid to boron nitride?
-
Suggest a reason why HF is a weak acid, whereas binary acids of the all other halogens are strong acids.
-
A solution of [Ni(H2O)6]2+ is green, whereas a solution of [Ni(CN)4]2- is colorless -Explain
-
The rate constant for a first order reaction is 1.54 x 10-3 s-1. Calculate its half life time.
-
The rate constant of a reaction at 400 and 200K are 0.04 and 0.02 s-1 respectively. Calculate the value of activation energy.
-
Calculate i) degree of hydrolysis, ii) the constant hydrolysis and iii) pH of 0.1M CH3COONa solution (pKa for CH3COOH is 4.74).
-
A copper electrode is dipped in 0.1M copper sulphate solution at 25oC. Calculate the electrode potential of copper. [Given: E0Cu2+|Cu = 0.34V].
-
Comment on the statement: Colloid is not a substance but it is a state of substance.
-
What is the difference between homogenous and hetrogenous catalysis?
-
Complete the following reactions
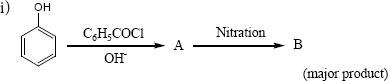
ii) \(C_6H_5-CH_{2}CH(OH)CH(CH_3)_2 \overset{ConH_2SO_4}\longrightarrow\) -
How would you distinguish between (i) methyl alcohol and ethyl alcohol (ii) benzyl alcohol and phenol, (iii) ethyl alcohol and benzyl alcohol?
-
Compound A with molecular formula C7H6O reduces Tollen's reagent and also gives Cannizaro reaction. A on oxidation gives the compound B with molecular formula C7H6O2 Calcium salt of B on dry distillation gives the compound C with molecular formula CI3H10O. Find A, B and C. Explain the reaction.
-
An organic compound (A) of molecular formula C7H6O is called as oil of bitter almonds. (A) on oxidation gives (B) of molecular formula C7H6O2 which gives brisk effervescence with NaHCO3 solution. When (A) is refluxed with aqueous alcoholic (KCN) compound (C) is formed. Identify A, B and C and write the equations.
-
A dibromo derivative (A) on treatment with KCN followed by acid hydrolysis and heating gives a monobasic acid (B) along with liberation of CO2 . (B) on heating with liquid ammonia followed by treating with Br2 /KOH gives (c) which on treating with NaNO2 and HCl at low temperature followed by oxidation gives a monobasic acid (D) having molecular mass 74. Identify A to D.
-
How do primary, secondary and tertiary amines react with nitrous acid?
-
What are different types of RNA which are found in cell
-
Elucidate the structure of glucose.
-
Explain the prepareation of Bakelite and Give its use.
20 x 5 = 100






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Chemistry - Full Portion Five Marks Questions
Write your Comment