- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Chemistry - Organic Nitrogen Compounds Model Question Paper Question Bank Software Oct-30 , 2019
Organic Nitrogen Compounds
Organic Nitrogen Compounds Model Question Paper
12th Standard
-
Reg.No. :
Chemistry
Time :
00:45:00 Hrs
Total Marks :
35
-
The method by which aniline cannot be prepared is _________.
(a)degradation of benzamide with Br2 / NaOH
(b)potassium salt of phthalimide treated with chlorobenzene followed by hydrolysis with aqueous NaOH solution.
(c)reduction of Nitrobenzene with LiAlH4
(d)reduction of nitrobenzene by Sn / HCl
-
CH3CH2 Br \(\overset { aqNaOH }{ \underset { \Delta }{ \longrightarrow } } A\overset { { KMnO }_{ 4 }{ /H }^{ + } }{ \underset { \Delta }{ \longrightarrow } } B\overset { { NH }_{ 3 } }{ \underset { \Delta }{ \longrightarrow } } C\overset { { Br }_{ 2 }/NaOH }{ \longrightarrow } D\) D' is________.
(a)bromomethane
(b)α - bromo sodium acetate
(c)methanamine
(d)acetamide
-
Which one of the following nitro compounds does not react with nitrous acid.
(a)CH3 -CH2 -CH2 -NO2
(b)(CH3)2 CH - CH2NO2
(c)(CH3)3 C NO2
(d)\({ CH }_{ 3 }-\underset { \overset { || }{ O } }{ C } -\underset { \overset { || }{ { CH }_{ 3 } } }{ CH } -{ NO }_{ 2 }\)
-
The product formed by the reaction an aldehyde with a primary amine ________.
(a)carboxylic acid
(b)aromatic acid
(c)schiff ’s base
(d)ketone
-
When aniline reacts with acetic anhydride the product formed is_______.
(a)o – aminoacetophenone
(b)m-aminoacetophenone
(c)p – aminoacetophenone
(d)acetanilide
-
There are two isomers with the formula CH3NO2. How will you distinguish between them?
-
How will you convert nitrobenzene into
i. 1,3,5 - trinitrobenzene
ii. o and p- nitrophenol
iii. m – nitro aniline
iv. azoxybenzene
v. hydrozobenzene
vi. N – phenylhydroxylamine
vii. aniline -
Identify compounds A, B and C in the following sequence of reactions.
i) \({ C }_{ 6 }{ H }_{ 5 }NO_{ 2 }\overset { Fe/HCL }{ \longrightarrow } A\overset { HN{ O }_{ 2 } }{ \underset { 273K }{ \longrightarrow } } B\overset { { C }_{ 6 }{ H }_{ 5 }OH }{ \longrightarrow } C\)
ii) \({ C }_{ 6 }{ H }_{ 5 }N_{ 2 }cl\overset { CuCN }{ \longrightarrow } A\overset { H_{ 2 }O/H^{ + } }{ \longrightarrow } B\overset { NH_3 }{ \longrightarrow } C\)
iii) \({ C }{ H }_{ 3 }{ C }{ H }_{ 2 }I\overset { NaCN}{ \longrightarrow } A\overset {OH^-}{ \underset {Partial hydrolysis}{ \longrightarrow } } B\overset {NaOH+Br_2 }{ \longrightarrow } C\)
iv) \({ C }{ H }_{ 3 }NH_{ 2 }\overset { CH_3 Br }{ \longrightarrow } A\overset { CH_{ 3 }COCl}{ \longrightarrow } B\overset { B_2H_6 }{ \longrightarrow } C\)
v) \({ C }_{ 6 }{ H }_{ 5 }NH_{ 2 }\overset { (CH_{ 3 }CO)_{ 2 }O }{ \underset { Pyridine }{ \longrightarrow } } A\overset { HNO_{ 3 } }{ \underset { H_{ 2 }SO_{ 4 },288K }{ \longrightarrow } } B\overset { { H }_{ 2 }O/{ H }^{ + } }{ \longrightarrow C } \)
vi)
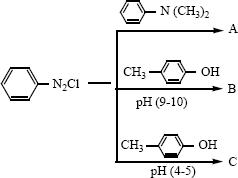
vii) \({ C }{ H }_{ 3 }CN_{ 2 }NC\overset { HgO }{ \longrightarrow } A\overset { H_{ 2 }O }{ \longrightarrow } B\overset { i) NaN{ O }_{ 2 }/HCL }{ \underset { ii){ H }_{ 2 }O }{ \longrightarrow } } \) -
How will you distinguish between primary secondary and tertiary alphatic amines.
-
Account for the following
i. Aniline does not undergo Friedel – Crafts reaction
ii. Diazonium salts of aromatic amines are more stable than those of aliphatic amines
iii. pKb of aniline is more than that of methylamine
iv. Gabriel phthalimide synthesis is preferred for synthesising primary amines.
v. Ethylamine is soluble in water whereas aniline is not
vi. Amines are more basic than amides
vii.Although amino group is o – and p – directing in aromatic electrophilic substitution reactions, aniline on nitration gives a substantial amount of m – nitroaniline. -
How will you prepare propan – 1- amine from
i) butane nitrile
ii) propanamide
ii) 1- nitropropane -
Identify A,B and C
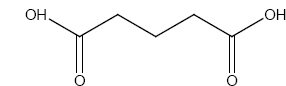 \(\overset{SOCl_2}\longrightarrow A \overset{NH_3}\longrightarrow B\overset{LiAlH_4}\longrightarrow (C)\)
\(\overset{SOCl_2}\longrightarrow A \overset{NH_3}\longrightarrow B\overset{LiAlH_4}\longrightarrow (C)\) -
A dibromo derivative (A) on treatment with KCN followed by acid hydrolysis and heating gives a monobasic acid (B) along with liberation of CO2 . (B) on heating with liquid ammonia followed by treating with Br2 /KOH gives (c) which on treating with NaNO2 and HCl at low temperature followed by oxidation gives a monobasic acid (D) having molecular mass 74. Identify A to D.
-
Identify A to E in the following sequence of reactions
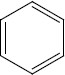
\(\overset { {CH_3} {CL} }{ \underset { {AlCl}_3 }{ \longrightarrow } }\) A \(\overset { {HNO_3}/ {H_2So_4} }{ \underset { {} }{ \longrightarrow } }\) B \(\overset { {Sn} /{HCL} }{ \underset {{} }{ \longrightarrow } }\) (C) \(\overset { {NaNo_2}/ {HCL} }{ \underset { {O^o}C }{ \longrightarrow } }\) D \(\overset { {CuCN} }{ \underset { {}}{ \longrightarrow } }\) E
5 x 1 = 5
3 x 2 = 6
3 x 3 = 9
3 x 5 = 15






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Chemistry - Organic Nitrogen Compounds Model Question Paper
Write your Comment