- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium Free Online Test 1 Mark Questions 2020 - Part Six Question Bank Software Oct-13 , 2020
12th Standard Chemistry English Medium Free Online Test 1 Mark Questions 2020 - Part Six
12th Standard Chemistry English Medium Free Online Test 1 Mark Questions 2020 - Part Six
12th Standard
-
Reg.No. :
Chemistry
Time :
00:25:00 Hrs
Total Marks :
25
-
The incorrect statement among the following is______.
(a)Nickel is refined by Mond’s process
(b)Titanium is refined by Van Arkel’s process
(c)Zinc blende is concentrated by froth floatation
(d)In the metallurgy of gold, the metal is leached with dilute sodium chloride solution
-
In Hall-Heroult process ___________act as an anode.
(a)Carbon blocks
(b)hydrogen
(c)copper rods
(d)Zinc rods
-
Silicones are ______.
(a)ortho silicates
(b)water repellent thermal insulators
(c)both (a) and (b)
(d)None of these
-
An element belongs to group 15 and 3rd period of the periodic table, its electronic configuration would be_______.
(a)1s2 2s2 2p4
(b)1s2 2s2 2p3
(c)1s2 2s2 2p6 3s2 3p2
(d)1s2 2s2 2p6 3s2 3p3
-
Which one of the following is not correct?
(a)La(OH)3 is less basic than Lu(OH)3
(b)In lanthanoid series ionic radius of Ln3+ ions decreases
(c)La is actually an element of transition metal series rather than lanthanide series
(d)Atomic radii of Zr and Hf are same because of lanthanide contract
-
_________ is known as Bayer's reagent.
(a)Hot dilute alkaline KMnO4
(b)Cold dilute alkaline KMnO4
(c)Hot Conc. acidic KMnO4
(d)Cold Conc. acidic KMnO4
-
Choose the correct statement.
(a)Square planar complexes are more stable than octahedral complexes
(b)The spin only magnetic moment of [Cu(Cl)4]2- is BM and it has square planar structure.
(c)Crystal field splitting energy \(\left( { \Delta }_{ 0 } \right) \) [FeF6]4- is higher than the \((\Delta _{ 0 })\) of [Fe(CN)6]4-
(d)crystal field stabilization energy of [V(H2O)6]2+ is higher than the crystal field stabilization of [Ti(H2O)6]2+
-
The oxidation state of the central metal ion in the complex [Co(H2O)(CN)(en)2]2+ is________.
(a)0
(b)+1
(c)+2
(d)+3
-
A two dimensional solid pattern formed by two different atoms X and Y is shown below. The black and white squares represent atoms X and Y respectively. The simplest formula for the compound based on the unit cell from the pattern is _______.
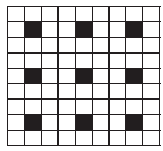 (a)
(a)XY8
(b)X4Y9
(c)XY2
(d)XY4
-
Graphite is a good conductor of electricity due to the presence of_______.
(a)Lone pair of electrons
(b)Free valence electrons
(c)Cations
(d)Anions
-
The correct difference between first and second order reactions is that________.
(a)A first order reaction can be catalysed; a second order reaction cannot be catalysed.
(b)The half life of a first order reaction does not depend on [A0]; the half life of a second order reaction does depend on [A0].
(c)The rate of a first order reaction does not depend on reactant concentrations; the rate of a second order reaction does depend on reactant concentrations.
(d)The rate of a first order reaction does depend on reactant concentrations; the rate of a second order reaction does not depend on reactant concentrations.
-
Which of the following statement is not correct?
(a)Molecularity of a reaction cannot be fractional
(b)Molecularity of a reaction cannot be more than three
(c)Molecularity of a reaction can be zero
(d)Molecularity is assigned for each elementary step of mechanism.
-
MY and NY3, are insoluble salts and have the same Ksp values of 6.2 × 10-13 at room temperature. Which statement would be true with regard to MY and NY3?
(a)The salts MY and NY3 are more soluble in 0.5M KY than in pure water
(b)The addition of the salt of KY to the suspension of MY and NY3 will have no effect on their solubility’s
(c)The molar solubility of MY and NY3 in water are identical
(d)The molar solubility of MY in water is less than that of NY3
-
For two acids A and B, Ka values at 25°C are 2 x 106 and 1.8 x 10-4 respectively. which among the following is true with respect to the above data _______.
(a)A and B are equally acidic
(b)A is stronger than B
(c)B is stronger than A
(d)Ka value is not a measure of acid strength
-
Which of the following electrolytic solution has the least specific conductance?
(a)2N
(b)0.002N
(c)0.02N
(d)0.2N
-
An example for 1 : 1 electrolyte is ______.
(a)H2SO4
(b)Na2SO4
(c)NaCI
(d)Al2(SO4)3
-
The coagulation values in millimoles per litre of the electrolytes used for the coagulation of As2S3 are given below
(I) (NaCl) = 52
(II) ((BaCl2) = 0.69
(III) (MgSO4) = 0.22
The correct order of their coagulating power is ________.(a)III > II > I
(b)I > II > III
(c)I > III > II
(d)II > III > I
-
Which of the following interface cannot be obtained?
(a)solid - solid
(b)solid - liquid
(c)gas - gas
(d)liquid -Iiquid
-
The process of removing ions from a sol by diffusion through a permeable membrane is called _______.
(a)Ultra filtration
(b)Dialysis
(c)Eletrophoresis
(d)Cataphoresis
-
An example of lyophilic colloid is________.
(a)sulphur in water
(b)phosphorus in water
(c)starch
(d)all of these
-
It has no α- hydrogen ______.
(a)CH3CH2OH
(b)CH3- CH2- CH2- OH
(c)\({ CH }_{ 3 }-\underset { \overset { | }{ { CH }_{ 3 } } }{ CH } -OH\)
(d) -
Complete the reaction
(a)(b)(c)(d)none
-
Of the following, which is the product formed when cyclohexanone undergoes aldol condensation followed by heating?
(a)(b)(c)(d) -
The major product of the following reaction
(a)(b)(c)(d) -
Which of the following are epimers?
(a)D(+)-Glucose and D(+)-Galactose
(b)D(+)-Glucose and D(+)-Mannose
(c)Neither (a) nor (b)
(d)Both (a) and (b)
Answer all the questions
25 x 1 = 25






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium Free Online Test 1 Mark Questions 2020 - Part Six
Write your Comment