- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Two Question Bank Software Oct-13 , 2020
12th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Two
12th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Two
12th Standard
-
Reg.No. :
Chemistry
Time :
00:10:00 Hrs
Total Marks :
10
-
Which of the following is not true with respect to Ellingham diagram?
(a)Free energy changes follow a straight line. Deviation occurs when there is a phase change.
(b)The graph for the formation of CO2 is a straight line almost parallel to free energy axis.
(c)Negative slope of CO shows that it becomes more stable with increase in temperature.
(d)Positive slope of metal oxides shows that their stabilities decrease with increase in temperature.
-
The stability of +1 oxidation state increases in the sequence ________.
(a)Al < Ga < In < Tl
(b)Tl < In < Ga < Al
(c)In < Tl < Ga < Al
(d)Ga< In < Al < Tl
-
When copper is heated with conc HNO3 it produces ________.
(a)Cu(NO3)2, NO and NO2
(b)Cu(NO3)2 and N2O
(c)Cu(NO3)2 and NO2
(d)Cu(NO3)2 and NO
-
Which one of the following is not correct?
(a)La(OH)3 is less basic than Lu(OH)3
(b)In lanthanoid series ionic radius of Ln3+ ions decreases
(c)La is actually an element of transition metal series rather than lanthanide series
(d)Atomic radii of Zr and Hf are same because of lanthanide contract
-
Choose the correct statement.
(a)Square planar complexes are more stable than octahedral complexes
(b)The spin only magnetic moment of [Cu(Cl)4]2- is BM and it has square planar structure.
(c)Crystal field splitting energy \(\left( { \Delta }_{ 0 } \right) \) [FeF6]4- is higher than the \((\Delta _{ 0 })\) of [Fe(CN)6]4-
(d)crystal field stabilization energy of [V(H2O)6]2+ is higher than the crystal field stabilization of [Ti(H2O)6]2+
-
A two dimensional solid pattern formed by two different atoms X and Y is shown below. The black and white squares represent atoms X and Y respectively. The simplest formula for the compound based on the unit cell from the pattern is _______.
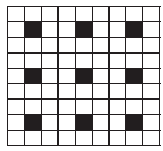 (a)
(a)XY8
(b)X4Y9
(c)XY2
(d)XY4
-
This reaction follows first order kinetics. The rate constant at particular temperature is 2.303 x 10-2 hour-1. The initial concentration of cyclopropane is 0.25 M. What will be the concentration of cyclopropane after 1806 minutes? (log 2 = 0.3010)
(a)0.125 M
(b)0.215 M
(c)0.25 x 2.303 M
(d)0.05 M
-
Dissociation constant of NH4OH is 1.8 x 10-5 the hydrolysis constant of NH4Cl would be _______.
(a)1.8 × 10-19
(b)5.55 × 10-10
(c)5.55 × 10-5
(d)1.80 × 10-5
-
Cell equation: A + 2B- \(\rightarrow\)A2++ 2B; A2+ + 2e- \(\rightarrow\)A Eo = +0.34V and log10 K = 15.6 at 300K for cell reactions find Eo for B+ + e− \(\rightarrow\) B
(a)0.80
(b)1.26
(c)-0.54
(d)-10.94
-
Match the following
a Pure nitrogen i Chlorine b Haber process ii Sulphuric acid c Contact process iii Ammonia d Deacons Process iv Sodium azide (or) Barium azide Which of the following is the correct option?
(a)A B C D i ii iii iv (b)A B C D ii iv i iii (c)A B C D iii iv ii i (d)A B C D iv iii ii i
Answer all the questions
10 x 1 = 10
*****************************************
Answers
-
(b)
The graph for the formation of CO2 is a straight line almost parallel to free energy axis.
-
(a)
Al < Ga < In < Tl
-
(c)
Cu(NO3)2 and NO2
-
(a)
La(OH)3 is less basic than Lu(OH)3
-
(d)
crystal field stabilization energy of [V(H2O)6]2+ is higher than the crystal field stabilization of [Ti(H2O)6]2+
-
(a)
XY8
-
(a)
0.125 M
-
(b)
5.55 × 10-10
-
(a)
0.80
-
(d)
A B C D iv iii ii i






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Two
Write your Comment