- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium Important 2 Mark Book Back Questions (New Syllabus) 2020 Question Bank Software Aug-31 , 2020
12th Standard Chemistry English Medium Important 2 Mark Book Back Questions (New Syllabus) 2020
Important 2 Mark Book Back Questions (New Syllabus) 2020
12th Standard
-
Reg.No. :
Chemistry
Time :
01:00:00 Hrs
Total Marks :
32
-
Boron does not react directly with hydrogen. Suggest one method to prepare diborane from BF3.
-
Explain why fluorine always exhibit an oxidation state of -1?
-
Justify the position of lanthanoids and actinoids in the periodic table.
-
[CuCl4]2- exists while [Cul4]2- does not exist why?
-
Explain briefly seven types of unit cell.
-
Describe the graphical representation of first order reaction.
-
What is the order with respect to each of the reactant and overall order of the following reactions?
a) 5Br-(aq)+BrO3-(aq)+6H+(aq) ➝3Br2(l)+3H2O(l)
The experimental rate law is Rate = k [Br−][BrO3−][H+]2
b) CH3CHO(g)\(\overset { \Delta }{ \longrightarrow } \) CH4(g)+CO(g) the experimental rate law is
Rate =K[CH3CHO]\(\frac{3}{2}\) -
The concentration of hydroxide ion in a water sample is found to be 2.5 × 10-6M. Identify the nature of the solution.
-
Why does conductivity of a solution decrease on dilution of the solution.
-
A conductivity cell has two platinum electrodes separated by a distance 1.5 cm and the cross sectional area of each electrode is 4.5 sq cm. Using this cell, the resistance of 0.5 N electrolytic solution was measured as 15 Ω. Find the specific conductance of the solution.
-
Differentiate physisorption and chemisorption.
-
Predict the major product, when 2-methyl but -2-ene is converted into an alcohol in each of the following methods.
(i) Acid catalysed hydration
(ii) Hydroboration
(iii) Hydroxylation using Baeyer's reagent -
State the laws of reflection.
-
Identify compounds A, B and C in the following sequence of reactions.
i) \({ C }_{ 6 }{ H }_{ 5 }NO_{ 2 }\overset { Fe/HCL }{ \longrightarrow } A\overset { HN{ O }_{ 2 } }{ \underset { 273K }{ \longrightarrow } } B\overset { { C }_{ 6 }{ H }_{ 5 }OH }{ \longrightarrow } C\)
ii) \({ C }_{ 6 }{ H }_{ 5 }N_{ 2 }cl\overset { CuCN }{ \longrightarrow } A\overset { H_{ 2 }O/H^{ + } }{ \longrightarrow } B\overset { NH_3 }{ \longrightarrow } C\)
iii) \({ C }{ H }_{ 3 }{ C }{ H }_{ 2 }I\overset { NaCN}{ \longrightarrow } A\overset {OH^-}{ \underset {Partial hydrolysis}{ \longrightarrow } } B\overset {NaOH+Br_2 }{ \longrightarrow } C\)
iv) \({ C }{ H }_{ 3 }NH_{ 2 }\overset { CH_3 Br }{ \longrightarrow } A\overset { CH_{ 3 }COCl}{ \longrightarrow } B\overset { B_2H_6 }{ \longrightarrow } C\)
v) \({ C }_{ 6 }{ H }_{ 5 }NH_{ 2 }\overset { (CH_{ 3 }CO)_{ 2 }O }{ \underset { Pyridine }{ \longrightarrow } } A\overset { HNO_{ 3 } }{ \underset { H_{ 2 }SO_{ 4 },288K }{ \longrightarrow } } B\overset { { H }_{ 2 }O/{ H }^{ + } }{ \longrightarrow C } \)
vi)
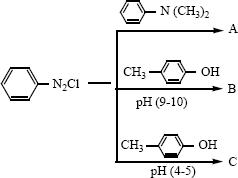
vii) \({ C }{ H }_{ 3 }CN_{ 2 }NC\overset { HgO }{ \longrightarrow } A\overset { H_{ 2 }O }{ \longrightarrow } B\overset { i) NaN{ O }_{ 2 }/HCL }{ \underset { ii){ H }_{ 2 }O }{ \longrightarrow } } \) -
Name the Vitamins whose deficiency cause i) rickets ii) scurvy
-
Write a note on synthetic detergents
Part A
16 x 2 = 32






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium Important 2 Mark Book Back Questions (New Syllabus) 2020
Write your Comment