- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard Chemistry English Medium - Solid State 1 Mark Book Back Question Paper and Answer Key 2022 - 2023 Study Materials Sep-01 , 2022
QB365 provides a detailed and simple solution for every Possible Book Back Questions in Class 12 Chemistry Subject - Solid State, English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
Solid State 1 Mark Book Back Question Paper With Answer Key
12th Standard
-
Reg.No. :
Chemistry
Time :
00:21:00 Hrs
Total Marks :
21
-
Graphite and diamond are ________.
(a)Covalent and molecular crystals
(b)ionic and covalent crystals
(c)both covalent crystals
(d)both molecular crystals
-
An ionic compound Ax By crystallizes in fcc type crystal structure with B ions at the centre of each face and A ion occupying corners of the cube the correct formula of Ax, By is ________.
(a)AB
(b)AB3
(c)A3B
(d)A8B6
-
The ratio of close packed atoms to tetrahedral hole in cubic packing is ________.
(a)1:1
(b)1:2
(c)2:1
(d)1:4
-
Solid CO2 is an example of ________.
(a)Covalent solid
(b)metallic solid
(c)molecular solid
(d)ionic solid
-
In calcium fluoride, having the fluorite structure the coordination number of Ca2+ ion and F- Ion are ________.
(a)4 and 2
(b)6 and 6
(c)8 and 4
(d)4 and 8
-
The number of unit cells in 8 gm of an element X (atomic mass 40) which crystallizes in bcc pattern is (NA is the Avogadro number)________.
(a)6.023 x 1023
(b)6.023 x 1022
(c)60.23 x 1023
(d)\(\left( \frac { 6.023\times { 10 }^{ 23 } }{ 8\times 40 } \right) \)
-
In a solid atom M occupies ccp lattice and \(\left( \frac { 1 }{ 3 } \right) \) of tetrahedral voids are occupied by atom N. Find the formula of solid formed by M and N ________.
(a)MN
(b)M3N
(c)MN3
(d)M3N2
-
The ionic radii of A+ and B− are 0.98 x 10-10 m and 1.81 x 10-10 m. the coordination number of each ion in AB is ________.
(a)8
(b)2
(c)6
(d)4
-
CsCl has bcc arrangement, its unit cell edge length is 400pm, its inter atomic distance is ________.
(a)400pm
(b)800pm
(c)\(\sqrt { 3 } \times 100pm\)
(d)\(\left( \frac { \sqrt { 3 } }{ 2 } \right) \times 400pm\)
-
A solid compound XY has NaCl structure if the radius of the cation is 100pm, the radius of the anion will be ________.
(a)\(\left( \frac { 100 }{ 0.414 } \right) \)
(b)\(\left( \frac { 0.732 }{ 100 } \right) \)
(c)100 x 0.414
(d)\(\left( \frac { 0.414 }{ 100 } \right) \)
-
The vacant space in bcc lattice unit cell is ________.
(a)48%
(b)23%
(c)32%
(d)26%
-
The radius of an atom is 300pm, if it crystallizes in a face centered cubic lattice, the length of the edge of the unit cell is ________.
(a)488.5pm
(b)848.5pm
(c)884.5pm
(d)484.5pm
-
The fraction of total volume occupied by the atoms in a simple cubic is ________.
(a)\(\left( \frac { \pi }{ 4\sqrt { 2 } } \right) \)
(b)\(\left( \frac { \pi }{ 6 } \right) \)
(c)\(\left( \frac { \pi }{ 4 } \right) \)
(d)\(\left( \frac { \pi }{ 3\sqrt { 2 } } \right) \)
-
The yellow colour in NaCl crystal is due to ________.
(a)excitation of electrons in F centers
(b)reflection of light from Cl- ion on the surface
(c)refraction of light from Na+ ion
(d)all of the above
-
If ‘a’ stands for the edge length of the cubic system sc, bcc, and fcc. Then the ratio of radii of spheres in these systems will be respectively ________.
(a)\(\left( \frac { 1 }{ 2 } a;\frac { \sqrt { 3 } }{ 2 } a;\frac { \sqrt { 2 } }{ 2 } a \right) \)
(b)\(\left( \sqrt { 1a } :\sqrt { 3a } :\sqrt { 2a } \right) \)
(c)\(\left( \frac { 1 }{ 2 } a:\frac { \sqrt { 3 } }{ 4 } a:\frac { 1 }{ 2\sqrt { 2 } } a \right) \)
(d)\(\frac { 1 }{ 2 } a:\sqrt { 3 } a:\frac { 1 }{ \sqrt { 2 } } a\)
-
If ‘a’ is the length of the side of the cube, the distance between the body centered atom and one corner atom in the cube will be_________.
(a)\(\left( \cfrac { 2 }{ \sqrt { 3 } } \right) a\)
(b)\(\left( \cfrac { 4 }{ \sqrt { 3 } } \right) a\)
(c)\(\left( \cfrac { \sqrt { 3 } }{ 4 } \right) a\)
(d)\(\left( \cfrac { \sqrt { 3 } }{ 2 } \right) a\)
-
Potassium has a bcc structure with nearest neighbor distance 4.52 \(\overset{o}{A}\). Its atomic weight is 39. its density will be_______.
(a)915 kg m-3
(b)2142 kg m-3
(c)452 kg m-3
(d)390 kg m-3
-
Schottky defect in a crystal is observed when ______.
(a)unequal number of anions and anions are missing from the lattice
(b)Equal number of cations and anions are missing from the lattice
(c)an ion leaves its normal site and occupies an interstitial site
(d)no ion is missing from its lattice
-
The cation leaves its normal position in the crystal and moves to some interstitial position, the defect in the crystal is known as _____.
(a)Schottky defect
(b)F center
(c)Frenkel defect
(d)non-stoichiometric defect
-
The crystal with a metal deficiency defect is ________.
(a)NaCl
(b)FeO
(c)ZnO
(d)KCl
-
A two dimensional solid pattern formed by two different atoms X and Y is shown below. The black and white squares represent atoms X and Y respectively. The simplest formula for the compound based on the unit cell from the pattern is _______.
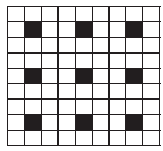 (a)
(a)XY8
(b)X4Y9
(c)XY2
(d)XY4
Multiple Choice Question
21 x 1 = 21
*****************************************
Answers
-
(c)
both covalent crystals
-
(b)
AB3
-
(b)
1:2
-
(c)
molecular solid
-
(c)
8 and 4
-
(b)
6.023 x 1022
-
(d)
M3N2
-
(c)
6
-
(d)
\(\left( \frac { \sqrt { 3 } }{ 2 } \right) \times 400pm\)
-
(a)
\(\left( \frac { 100 }{ 0.414 } \right) \)
-
(c)
32%
-
(b)
848.5pm
-
(b)
\(\left( \frac { \pi }{ 6 } \right) \)
-
(a)
excitation of electrons in F centers
-
(c)
\(\left( \frac { 1 }{ 2 } a:\frac { \sqrt { 3 } }{ 4 } a:\frac { 1 }{ 2\sqrt { 2 } } a \right) \)
-
(d)
\(\left( \cfrac { \sqrt { 3 } }{ 2 } \right) a\)
-
(a)
915 kg m-3
-
(b)
Equal number of cations and anions are missing from the lattice
-
(c)
Frenkel defect
-
(b)
FeO
-
(a)
XY8






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard Chemistry English Medium - Solid State 1 Mark Book Back Question Paper and Answer Key 2022 - 2023
Write your Comment