- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

12th Standard English Medium Chemistry Reduced Syllabus Annual Exam Model Question Paper - 2021 Question Bank Software Feb-27 , 2021
12th Standard English Medium Chemistry Reduced Syllabus Annual Exam Model Question Paper - 2021
12th Standard English Medium Chemistry Reduced Syllabus Annual Exam Model Question Paper - 2021
12th Standard
-
Reg.No. :
Chemistry
Time :
02:45:00 Hrs
Total Marks :
70
-
Sodium magnesium and aluminum can be obtained from their ore by________.
(a)electro metallurgy
(b)pyro metallurgy
(c)hydro metallurgy
(d)smelting
-
Oxidation state of carbon in its hydrides _______.
(a)+4
(b)-4
(c)+3
(d)+2
-
S-S bond is present in _______.
(a)H2S2O7
(b)H2SO5
(c)H2S2O6
(d)H2S2O6
-
The alloy of copper that contain Zinc is________.
(a)Monel metal
(b)Bronze
(c)bell metal
(d)brass
-
An excess of silver nitrate is added to 100ml of a 0.01M solution of Pentaaquachlorochromium (III)chloride. The number of moles of AgCl precipitated would be _______.
(a)0.02
(b)0.002
(c)0.01
(d)0.2
-
A binary solid A+B has a structure with B- ions constituting the lattice and A+ ions occupying 25% tetrahedral holes. Formula of the solid is _______.
(a)A2B
(b)AB2
(c)AB
(d)AB4
-
For a reaction Rate = k[acetone]3/2 then unit of rate constant and rate of reaction respectively is _______.
(a)(mol L-1 S-1),(mol1/2 L1/2 S-1)
(b)(mol-1/2 L1/2 s-1),(mol L-1 s-1)
(c)(mol1/2 L1/2 s-1),(mol L-1 s-1)
(d)(mol L s-1),(mol1/2 L1/2 s)
-
Which of the following fluro compounds is most likely to behave as a Lewis base?
(a)BF3
(b)PF3
(c)CF4
(d)SiF4
-
With regard to the strength of acids and bases, Find the incorrect statement among the following.
(a)Strong acid is one that completely dissociates in water
(b)Ka is the dissociation constant
(c)CH3COOH is a weak acid
(d)Smaller the Ka value, greater is the acid strength
-
The feasibility of a redox reaction can be predicted with the help of ______.
(a)Electronegativity
(b)Electrochemical series
(c)Electron affinity
(d)Equivalent conductance
-
The phenomenon observed when a beam of light is passed through a colloidal solution is_______.
(a)Cataphoresis
(b)Electrophoresis
(c)Coagulation
(d)Tyndall effect
-
Glycol \(\overset { 773K }{ \longrightarrow } \) 'A'. Identify 'A' _______.
(a)(b)\(\overset { { CH }_{ 3 } }{ \underset { CHO }{ | } } \)
(c)(d) -
Benzoic acid \(\overset { i){ NH }_{ 3 } }{ \underset { ii)\Delta }{ \longrightarrow } } A\overset { NaOBr }{ \longrightarrow } B\overset { NaN{ O }_{ 2 }/HCl }{ \longrightarrow } \) C 'C' is ______.
(a)anilinium chloride
(b)O – nitro aniline
(c)benzene diazonium chloride
(d)m– nitro benzoic acid
-
Oxidation of aniline with acidified potassium dichromate give
(a)p-benzo quinone
(b)benzoic acid
(c)benzaldehyde
(d)benzyl alcohol
-
The sugar unit present in nucleic acid is___________
(a)a hexose
(b)tetrose
(c)pentose
(d)glucose
-
Name the two steps involved in the extraction of crude metal
-
What is catenation ? describe briefly the catenation property of carbon.
-
Give the reason for bleaching action of Cl2.
-
Draw the Cis and trans isomer of MA2B2 type
-
Distinguish tetrahedral and octahedral voids.
-
If the rate of a reaction gets doubled as the temperature is increased from 27oC to 37oC. Find the activation energy of reaction?
-
When aqueous ammonia is added to CuSO4 solution, the solution turns deep blue due to the formation of tetra ammine copper (II) complex,\({ [Cu({ H }_{ 2 }O)_4] }_{ (aq) }^{ 2+ }+ 4{ NH }_{ 3 }(aq)\rightleftharpoons { [Cu{ ({ NH }_{ 3 }) }_{ 4 }] }_{ (aq) }^{ 2+ }\) among H2O and NH3 Which is stronger Lewis base.
-
Give the uses of mercury button cell.
-
Suggest a suitable reagent to prepare secondary alcohol with identical group using Grignard reagent.
-
What is liquation?
-
Account for the following:
(i) Cobalt (II) is stable in aqueous solution but in the presence of complexing reagents, it is easily oxidised.
(ii) The d1 configuration is very unstable in ions. -
Explain the process of recharging of lead storage battery.
-
What is the difference between a sol and a gel?
-
How is nitroglycerine prepared from glycerol?
-
How will you convert benzaldehyde into the following compounds?
(i) benzophenone
(ii) benzoic acid
(iii) α-hydroxyphenylaceticacid. -
Convert aniline to p-nitro aniline
-
Justify the presence of keto group in C2 in fructose.
-
Give the structure of melamine formaldehyde resin.
-
-
How will you convert boric acid to boron nitride?
-
Account for the following:
(i) Reducing character decreases from SO2 to TeO2.
(ii) Xenon forms compounds with fluorine and oxygen only.
-
-
-
Complete the following reactions
(i) Cr2O72- ⟶
(ii) Cr2O72- + 6I-+ 14H+ ⟶
(iii) Cr2O72-+ 3S2-+ 14H+ ⟶
(iv) Cr2O72- + 3SO2 + 2H+ ⟶
(v) Cr2O72- + 3Sn2+ + 14H+ ⟶
(vi) K2Cr2O7 + 8H2SO4 + 3CH3CH2OH ⟶
(vii) 2MnO4- + 5(COO)2- + 6H+ ⟶
(viii) 2MnO4- + 10I- + 16H+ ⟶
(ix) 2MnO4- + 5S2-+ 16H+ ⟶
(x) 2MnO4- + 5NO2- + 6H+ ⟶
(xi) 2KMnO4 + 3H2SO4 + 5CH3CH2OH ⟶
(xii) 2MnO4- + 5SO32- + 6H+ ⟶ -
What are the general characteristics of solids?
-
-
-
Give the structure for the following compounds.
(i) pentaamminechlorocobalt (III) ion
(ii) Triamminetrinitrito- k N cobalt (III)
(ill) tetraammineaquabromidooobalt(III)nitrate
(iv) Dichloridobisethane-(1,2-diamine) cobalt (lIl) chloride
(v) Tetraamminecopper (lI) sulphate -
Benzene diazonium chloride in aqueous solution decomposes according to the equation \({ C }_{ 6 }{ H }_{ 5 }{ N }_{ 2 }Cl\longrightarrow { C }_{ 6 }{ H }_{ 5 }Cl+{ N }_{ 2 }\)Starting with an initial concentration of 10g L-1, the volume of N2 gas obtained at 50 °C at different intervals of time was found to be as under:
t(min) 6 12 18 24 30 \(\infty \) Vol of N2 (ml) 19.3 32.6 41.3 46.5 50.4 58.3 Show that the above reaction follows the first order kinetics. What is the value of the rate constant?
-
-
-
Ksp of Al(OH)3 is 1\(\times\)10-15M. At what pH does 1.0×10-3M Al3+ precipitate on the addition of buffer of NH4Cl and NH4OH solution?
-
Explain the following
(i) Reactant selectivity
(ii) Transition state selectivity
(iii) Product selectivity
-
-
-
Complete the following reactions
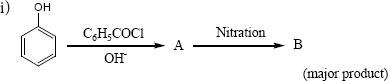
ii) \(C_6H_5-CH_{2}CH(OH)CH(CH_3)_2 \overset{ConH_2SO_4}\longrightarrow\) -
Compound A of molecular formula C3H6O does not reduce Tollens reagent and Fehling's solution. Compound A undergoes Clemmensen reduction to give compound B of molecular formula C3H8. Compound A in the presence of cone.H2SO4 condenses to give an aromatic compound C of molecular formula C9H12. Identify A, B and C. Explain the reactions.
-
Part I
Answer all the questions.
Choose the most suitable answer from the given four alternatives and write the option code with the corresponding answer.
15 x 1 = 15
Part II
Answer any 6 of the questions. Question no.24 is compulsory.
6 x 2 = 12
Part III
Answer any 6 of the questions. Question no.33 is compulsory.
6 x 3 = 18
Part IV
Answer all the questions.
5 x 5 = 25






 12th Standard Chemistry Syllabus
12th Standard Chemistry Syllabus  12th Standard Chemistry Study Materials
12th Standard Chemistry Study Materials 12th Standard Chemistry MCQ Practise Tests
12th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 12th Standard English Medium Chemistry Reduced Syllabus Annual Exam Model Question Paper - 2021
Write your Comment