- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Three Question Bank Software Nov-10 , 2020
11th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Three
11th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Three
11th Standard
-
Reg.No. :
Chemistry
Time :
00:10:00 Hrs
Total Marks :
10
-
When 22.4 litres of H2(g) is mixed with 11.2 litres of Cl2(g), each at 273 K at 1 atm the moles of HCl (g), formed is equal to ______.
(a)2 moles of HCI (g)
(b)0.5 moles of HCI (g)
(c)1.5 moles of HCI (g)
(d)1 moles of HCI (g)
-
For d-electron, the orbital angular momentum is ___________
(a)\(\frac { \sqrt { 2 } h }{ 2\pi } \)
(b)\(\\ \frac { \sqrt { 2h } }{ 2\pi } \)
(c)\(\frac { \sqrt { 2\times 4 } h }{ 2\pi } \)
(d)\(\frac { \sqrt { 6 } h }{ 2\pi } \)
-
Identify the wrong statement.
(a)Amongst the isoelectronic species, smaller the positive charge on cation, smaller is the ionic radius
(b)Amongst isoelectric species greater the negative charge on the anion, larger is the ionic radius
(c)Atomic radius of the elements increases as one moves down the first group of the periodic table
(d)Atomic radius of the elements decreases as one moves across from left to right in the 2nd period of the periodic table.
-
Zeolite used to soften hardness of water is hydrated _____________
(a)Sodium aluminium silicate
(b)Calcium aluminium silicate
(c)Zinc aluminium borate
(d)Lithium aluminium hydride
-
In a closed room of 1000 m3 a perfume bottle is opened up. The room develops smell. This is due to which property of gases _____________
(a)Viscosity
(b)Density
(c)Diffusion
(d)None
-
The values of ΔH and ΔS for a reaction are respectively 30 kJ mol-1 and 100 JK-1 mol-1. Then the temperature above which the reaction will become spontaneous is ______________
(a)300 K
(b)30 K
(c)100 K
(d)200 C
-
A 20 litre container at 400 K contains CO2 (g) at pressure 0.4 atm and an excess of SrO (neglect the volume of solid SrO). The volume of the container is now decreased by moving the movable piston fitted in the container. The maximum volume of the container, when pressure of CO2 attains its maximum value will be: Given that: SrCO3 (S) ⇌ SrO (S) + CO2(g)
KP = 1.6 atm(a)2 litre
(b)5 litre
(c)10 litre
(d)4 litre
-
Assertion: An ideal solution obeys Raoults Law
Reason: In an ideal solution, solvent-solvent, as well as solute-solute interactions, are similar to solute-solvent interactions.
a) both assertion and reason are true and reason is the correct explanation of assertion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) both assertion and reason are false -
The percentage of s-character of the hybrid orbitals in methane, ethane, ethene and ethyne are respectively ___________
(a)25, 25, 33, 3, 50
(b)50, 50, 33, 3, 25
(c)50, 25, 33, 3, 50
(d)50, 25, 25, 50
-
Assertion:
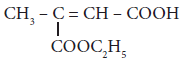 is 3– carbethoxy -2- butenoicacid
is 3– carbethoxy -2- butenoicacid
Reason: The principal functional group gets lowest number followed by double bond (or) triple bond.(a)both the assertion and reason are true and the reason is the correct explanation of assertion
(b)both assertion and reason are true and the reason is not the correct explanation of assertion
(c)assertion is true but reason is false
(d)both the assertion and reason are false
Answer all the questions
10 x 1 = 10
*****************************************
Answers
-
(d)
1 moles of HCI (g)
-
(d)
\(\frac { \sqrt { 6 } h }{ 2\pi } \)
-
(a)
Amongst the isoelectronic species, smaller the positive charge on cation, smaller is the ionic radius
-
(a)
Sodium aluminium silicate
-
(c)
Diffusion
-
(a)
300 K
-
(b)
5 litre
-
(a)
25, 25, 33, 3, 50
-
(a)
both the assertion and reason are true and the reason is the correct explanation of assertion






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium Free Online Test Book Back 1 Mark Questions with Answer Key - Part Three
Write your Comment