- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium - Important 2 Mark Question Paper and Answer Key 2022 - 2023 Study Materials Dec-31 , 2022
QB365 provides a detailed and simple solution for every Possible Questions in Class 11 Chemistry Subject - Important 2 Mark English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
11th Standard Chemistry Important 2 Mark Question with Answers
11th Standard
-
Reg.No. :
Chemistry
Time :
01:30:00 Hrs
Total Marks :
60
-
Define relative atomic mass.
-
Calculate the average atomic mass of naturally occurring magnesium using the following data.
Isotope Istopic atomic mass Abundance(%) Mg24 23.99 78.99 Mg25 24.99 10.00 Mg26 25.98 11.01 -
Consider the following electronic arrangements for the d5 configuration.
(a)\(\upharpoonleft \downharpoonright \) \(\upharpoonleft \downharpoonright \) \(\upharpoonleft \) (b)
\(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \downharpoonright \) (c)
\(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \) \(\upharpoonleft \) which of these represents the ground state
-
Which ion has the stable electronic configuration? Ni2+ or Fe3+.
-
What are isoelectronic ions? Give examples.
-
The first ionisation energy (lE1) and second ionisation energy (lE2) of elements X, Y and Z are given below.
Element IE1(kJ mol-1) IE2(kJ mol-1) X 2370 5250 Y 522 7298 Z 1680 3381 Which one of the above elements is the most reactive metal, the least reactive metal and a noble gas?
-
Why sodium hydroxide is much more water soluble than chloride ?
-
Give suitable explanation for the following facts about gases.
Gases don't settle at the bottom of a container. -
Define Hess's law of constant heat summation.
-
Calculate ΔHr0 for the reaction CO2(g) + H2(g) ⟶ CO(g) + H2O (g) given that ΔHf0 for CO2 (g), CO (g) and H2O (g) are - 393.5, - 111.31 and - 242 kJ mol-1 respectively.
-
Consider the following reactions,
H2(g) + I2(g) ⇌ 2 HI(g)
In each of the above reaction find out whether you have to increase (or) decrease the volume to increase the yield of the product. -
Consider the following reactions,
CaCO3 (s) ⇌ CaO (s) + CO2(g)
In the above reaction find out whether you have to increase (or) decrease the volume to increase the yield of the product. -
If 5.6 g of KOH is present in
(a) 500 mL and
(b) 1 litre of solution
Calculate the molarity of each of these solutions. -
Define the term ‘isotonic solution’.
-
Draw the lewis structure for
Phosphoric acid -
Draw the MO diagram for acetylide ion C22– and calculate its bond order.
-
Classify the following compounds based on the structure
\(\text { i) } \mathrm{CH} \equiv \mathrm{C}-\mathrm{CH}_{2}-\mathrm{C} \equiv \mathrm{CH}\)
\(\text { ii) } \mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{CH}_{3}\)
-
Give two examples for each of the following type of organic compounds.
aliphatic open chain. -
Write short notes on Resonance.
-
How will you prepare propane from a sodium salt of fatty acid?
-
Write the IUPAC names for the following alkenes.
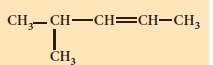
-
Give IUPAC names for the following compound
(CH3)3 C – C ≡ C – CH (CH3)2 -
Draw the structures for the following alkenes.
4 – Methyl – 2 pentene -
Discuss the aromatic nucleophilic substitutions reaction of chlorobenzene.
-
Write the equilibrium constant (KC) expression for the following reactions
(i) Cu2+(aq) + 2Ag (s) ⇌ Cu(s) + 2Ag+ (aq)
(ii) 4HCl(g) + O2g ⇌ 2Cl2(g) + 2H2O(g) -
State Henry's law.
-
How does the polarizing power of cations affect the covalent character imparted into the ionic bond ?
-
Calculate the formal charge of the atoms (numbered) in the following:
-
Expand the structure.
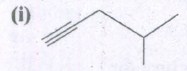
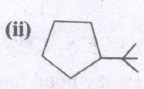
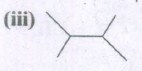
-
How will you control photochemical smog?
Answer All The Questions
30 x 2 = 60
*****************************************
Answers






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium - Important 2 Mark Question Paper and Answer Key 2022 - 2023
Write your Comment