- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium Free Online Test Creative 1 Mark Questions - Part Two Question Bank Software Nov-10 , 2020
11th Standard Chemistry English Medium Free Online Test Creative 1 Mark Questions - Part Two
11th Standard Chemistry English Medium Free Online Test Creative 1 Mark Questions - Part Two
11th Standard
-
Reg.No. :
Chemistry
Time :
00:10:00 Hrs
Total Marks :
10
-
Which of the following statement(s) is/are not true about the following decomposition reaction.
2KClO3 \(\longrightarrow\) 2KCl + 3O2
(i) Potassium is undergoing oxidation
(ii) Chlorine is undergoing oxidation
(iii) Oxygen is reduced
(iv) None of the species are undergoing oxidation and reduction.(a)only (iv)
(b)(i) and (iv)
(c)(iv) and (iii)
(d)All of these
-
Which of the following provides the experimental justification of magnetic quantum number?
(a)Zeeman effect
(b)Stark effect
(c)Uncertainty principle
(d)Quantum condition
-
Group 16 constitutes_____family.
(a)halogen
(b)nobel gas
(c)chalcogen
(d)alkali metals
-
Hydrogen combines with carbon monoxide in the presence of copper catalyst will synthesise _____________
(a)Ethanol
(b)Methane
(c)Methanol
(d)Methanal
-
Match the list I with List II and select the correct answer using. the code given below the lists.
List I List II A Gypsum 1 Bleaching Powder B Plaster of paris 2 Chlorophyll C Slaked lime 3 Statues D Magnesium 4 Satin spar (a)A B C D 1 3 2 4 (b)A B C D 4 3 1 2 (c)A B C D 3 4 1 2 (d)A B C D 2 1 4 3 -
Which one of the following is the formula of limestone?
(a)CaO
(b)Ca(OH)2
(c)CaCO3
(d)CaCO3.MgCO3
-
The isotherm obtained for CO is as follows:
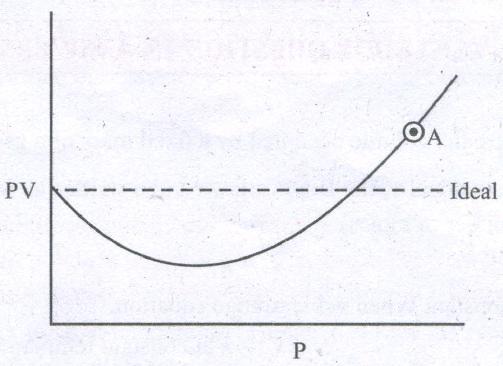
The compressibility factor for the gas at point ' A' will be __________(a)\((1-\frac{b}{V})\)
(b)\((1+\frac{b}{V})\)
(c)\((1+\frac{b}{RT})\)
(d)\((1+\frac{a}{RTV})\)
-
The change in enthalpy of NaOH + HCl ⟶ NaCI + H2O is called _________
(a)Heat of reaction
(b)Heat of neutralization
(c)Heat of formation
(d)Heat of liquid
-
The standard substance used in the enthalpy of combustion of a substance in bomb calorimeter is _______
(a)methane
(b)acetic acid
(c)propane
(d)benzoic acid
-
The value of equilibrium constant of the reaction,
\({ HI }_{ \left( g \right) }\rightleftharpoons \cfrac { 1 }{ 2 } { H }_{ 2\left( g \right) }+\cfrac { 1 }{ 2 } { I }_{ 2\left( g \right) }\) is 8.0. The equilibrium constant of the reaction; \({ H }_{ 2\left( g \right) }+I_{ 2\left( g \right) }\rightleftharpoons { 2HI }_{ \left( g \right) }\)(a)\(\cfrac { 1 }{ 8 } \)
(b)\(\cfrac { 1 }{ 16 } \)
(c)16
(d)\(\cfrac { 1 }{ 64 } \)
Answer all the questions
10 x 1 = 10






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium Free Online Test Creative 1 Mark Questions - Part Two
Write your Comment