- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium - Important 5 Mark Question Paper and Answer Key 2022 - 2023 Study Materials Dec-31 , 2022
QB365 provides a detailed and simple solution for every Possible Questions in Class 11 Chemistry Subject - Important 5 Mark English Medium. It will help Students to get more practice questions, Students can Practice these question papers in addition to score best marks.
11th Standard Chemistry Important 5 Mark Question with Answers
11th Standard
-
Reg.No. :
Chemistry
Time :
02:30:00 Hrs
Total Marks :
150
-
Mass of one atom of an element is 6.645 x 10-23g. How many moles of element are there in 0.320 kg.
-
Balance the following equations by ion electron method.
i) \({ KMn }O_{ 4 }+{ SnCl }_{ 2 }+HCI\longrightarrow MnCI_{ 2 }+{ SnCI }_{ 4 }+{ H }_{ 2 }O+KCI\)
ii)
iii)
iv) -
Balance the following equation using oxidation number method
As2 S3 + HNO3 + H2O \(\rightarrow\) H3 AsO4 + H2 SO4 + NO -
State and explain pauli exclusion principle.
-
i) Describe the Aufbau principle.
ii) What is effective nuclear charge ? -
Explain the pauling method for the determination of ionic radius.
-
How do you convert para hydrogen into ortho hydrogen?
-
Explain the important common features of Group 2 elements.
-
Distinguish between diffusion and effusion.
-
Define the following terms
(a) isothermal process (b) adiabatic process
(c) isobaric process (d) isochoric process -
List the characteristics of Gibbs free energy
-
Calculate the enthalpy change for the reaction
Fe2O3 + 3CO ⟶ 2Fe + 3CO2 from the following data.
2Fe +\(\frac{3}{2}\)O2 ⟶ Fe2O3; ΔH = -741 kJ
C +\(\frac{1}{2}\)O2 ⟶ CO; ΔH = -137 kJ
C + O2 ⟶ CO2; ΔH = - 394.5 kJ -
When I-pentyne (A) is treated with 4N alcoholic KOH at 175°C, it is converted slowly into an equilibrium mixture of 1.3% I-pentyne(A) , 95.2% 2-pentyne(B) and 3.5% of 1,2 pentadiene (C) the equilibrium was maintained at 175°C, calculate ΔG0 for the following equilibria.
B \(\rightleftharpoons \)AGIO?
B \(\rightleftharpoons \)CG20? -
The equilibrium constant at 298 K for a reaction is 100.
A + B \(\rightleftharpoons \) C + D
If the initial concentration of all the four species is 1 M, the equilibrium concentration of D (in mol lit-1) will be -
State and explain Henry’s law.
-
Explain resonance with reference to carbonate ion ?
-
Explain VSEPR theory. Applying this theory to predict the shapes of IF7, and SF6.
-
Describe the reactions involved in the detection of nitrogen in an organic compound by Lassaigne method.
-
Identify the compound A, B, C and D in the following series of reactions
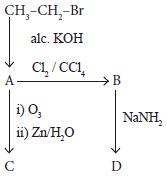
-
Differentiate the following
(i) BOD and COD
(ii) Viable and non-viable particulate pollutants -
Arrange the elements silver, Zinc and copper in the order of their decreasing electron releasing tendency and justify your arrangement with an appropriate experiment.
-
Balance the following equations by oxidation number method.
P + HNO3 ⟶ HPO3 + NO + H2O -
Give the chemical properties of heavy water and water.
-
Explain the preparation and uses of the following compounds of calcium.
-
Explain P-V relationship experiments of Robert Boyle.
-
If a scuba diver takes a breath at the surface filling his lungs with 5.82 dm3 of air what volume will the air in his lungs occupy when he drives to a depth where the pressure is 1.92 atm. (assume temperature is constant and the pressure at the surface is exactly)
-
At sea level a balloon has volume of 785 x10-3dm3 What will be its volume, if it taken to a place where the pressure is 0052 atm. Less than the atmospheric pressure of 1 atm.
-
The critical temperature of hydrogen gas is 33.2 °C and its critical pressure is 12.4 atm. Find out the values of a and b.
-
Explain Andrew's isotherm of carbon dioxide.
-
Calculate the entropy change in the engine that receives 957.5 kJ of heat reversibly at 110°C temperature.
Answer All The Questions
30 x 5 = 150
*****************************************
Answers






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium - Important 5 Mark Question Paper and Answer Key 2022 - 2023
Write your Comment