- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

Haloalkanes and Haloarenes Important Questions Dec-14 , 2018
HSC Board Exams are fast approaching and students are getting anxious about how to prepare for their HSC Board Exams. The latest sample papers have been designed as per the latest syllabus and examination trends. Students can make use of this question paper since all the questions are taken from the book back and creative question papers. Our aim is to bring out the best scores on board exams. By practicing with this important model question paper student can gain more knowledge and confidence to crack the exams.
In this question paper, all the important questions from 11th Chemistry Chapter Haloalkanes and Haloarenes are included for practice. Not only with book back questions, but also included creative questions. It's nearing the examination and right time to grab the model question papers and practice for the board exams. Everyone has the same aim that is to reach the top rank list. We are paving the way for you to reach your goal.
Practice with important questions from all chapters and get set ready to crack your exams with confidence. Concentrate on making small improvements and importantly keep practicing model question papers. This question paper exclusively designed for the chapter Haloalkanes and Haloarenes from plus two Chemistry. Since board exams are very important for your upcoming career, you should pay attention to practice and score good marks. The best way to prepare exams is to simply do one thing at a time. Now you can use this model question paper for practice on your own to score more on your board exams.
Haloalkanes and Haloarenes Important Questions
11th Standard
-
Reg.No. :
Chemistry
Time :
01:00:00 Hrs
Total Marks :
50
-
Of the following compounds, which has the highest boiling point?
(a)n-Butyl chloride
(b)Isobutyl chloride
(c)t-Butyl chloride
(d)n-propyl chloride
-
In the reaction
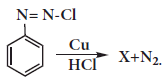 X is ________.(a)
X is ________.(a) (b)
(b) (c)
(c) (d)
(d)
-
The name of C2F4Cl2 is ___________
(a)Freon – 112
(b)Freon – 113
(c)Freon – 114
(d)Freon – 115
-
Freon-12 is manufactured from tetrachloro methane by ____________
(a)Wurtz reaction
(b)Swarts reaction
(c)Haloform reaction
(d)Gattermann reaction
-
Silverpropionate when refluxed with Bromine in carbontetrachloride gives ______
(a)propionic acid
(b)chloro ethane
(c)bromo ethane
(d)chloro propane
-
The best reagent for converting an alcohol into the corresponding chloride is
(a)PCl3
(b)PCl5
(c)SOCl2
(d)HCl/ZnCl2
-
Ethyl bromide reacts with sodium lead alloy to form_____________.
(a)Chloro ethane
(b)1 - Chluro propane
(c)Vinyl chloride.
(d)Bromo benze
-
For reacting with HCI, the alcohol which does not require ZnCl2 is ____________
(a)CH3 CH2 OH
(b)CH3-CH2 CH2 OH
(c)CH3-CH OH
(d)(CH3)3 C - OH
-
Mild oxidation of benzyl chloride with Cu(NO3)Z gives __________
(a)Benzoic acid
(b)Benzene
(c)benzaldehyde
(d)Benzyl alcohol
-
Chloropicrin is prepared by adding nitric acid to______________,
(a)CCl4
(b)CH3CI
(c)CH3CI3
(d)None
-
Why chlorination of methane is not possible in dark?
-
Write down the possible isomers of C5H11Br and give their IUPAC and common names.
-
Explain the preparation of the following compounds
i) DDT
ii) Chloroform
iii) Biphenyl
iv) Chloropicrin
v) Freon-12 -
Write a note on carbylamine reaction.
-
Write the isomers of the compound whose molcular formula is C4H9Br.
-
An organic compound (A) with molecular formula C2H5Cl reacts with KOH gives compounds (B) and with alcoholic KOH gives compound (C). Identify (A),(B), and (C)
-
Two isomers (A) and (B) have the same molecular formula C2H4Cl2. Compound (A) reacts with aqueous KOH gives compound (C) of molecular formula C2H4O. Compound (B) reacts with aqueous KOH gives compound(D) of molecular formula C2H6O2. Identify (A), (B), (C) and (D).
-
Haloalkanes produce mixture of olefins- say true or false and justify yoyr answer,
-
Among ortho, meta and para substituted diahalobenzenes which has high melting point? Give reason with example.
-
Ahydroarbon C5H10 does not react with chlorine In dark but gives C5H9CI in bright sunlight. Identify the hydrocarbon.
-
Explain the mechanism involved in bimolecular nucleophilic substitution reaction.
-
How will you convert t-butyl bromide into t-butyl alcohol? Explain the process through the mechanism in stepwise manner.
-
Answer the following.
(i) Predict the major product formed when HCI is added to iso-butylene,
(ii) What happens when CH3-Br is treated with KCN?
(iii) Identify the chiral molecule in the following pair.
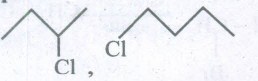
(iv) Arrange the compounds in the order of I reactivity towards SN2 displacement. 2-Bromo-2-methylbutane, I-Bromopentane, 2-Bromo pentane.
Part A
10 x 1 = 10
Part B
5 x 2 = 10
Part C
5 x 3 = 15
Part D
3 x 5 = 15






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about Haloalkanes and Haloarenes Important Questions
Write your Comment