- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students
Half Yearly Model Question Paper 2019
11th Standard
-
Reg.No. :
Chemistry
Time :
02:30:00 Hrs
Total Marks :
70
-
40 ml of methane is completely burnt using 80 ml of oxygen at room temperature The volume of gas left after cooling to room temperature is _______.
(a)40 ml CO2 gas
(b)40 ml CO2 gas and 80 ml H2O gas
(c)60 ml CO2 gas and 60 ml H2O gas
(d)120 ml CO2 gas
-
Two electrons occupying the same orbital are distinguished by ___________
(a)azimuthal quantum number
(b)spin quantum number
(c)magnetic quantum number
(d)orbital quantum number
-
Law of triad was unable to explain for the element
(a)Ca, Sr and Ba
(b)Fe, Co, Ni
(c)Li, Na, K
(d)CI, Br, I
-
Heavy water is used as _________
(a)moderator in nuclear reactions
(b)coolant in nuclear reactions
(c)both (a) and (b)
(d)none of these
-
The suspension of slaked lime in water is known as ___________
(a)lime water
(b)quick lime
(c)milk of lime
(d)aqueous solution of slaked lime
-
The ideal gas equation is ____________.
(a)PV = RT for 1 mole
(b)P1V1 = P2V2
(c)\(\frac{P}{T}=R\)
(d)P = P1 + P2 + P3
-
An ideal gas expands from the volume of 1 x 10-3 m3 to 1 x 10-2 m3 at 300 K against a constant pressure at 1 x 105 Nm-2. The work done is ______________
(a)- 900 J
(b)900 kJ
(c)270 kJ
(d)-900 kJ
-
Solubility of carbon dioxide gas in cold water can be increased by ____________
(a)increase in pressure
(b)decrease in pressure
(c)increase in volume
(d)none of these
-
The KH for the solution of oxygen dissolved in water is 4\(\times\)104 atm at a given temperature. If the partial pressure of oxygen in air is 0.4 atm, the mole fraction of oxygen in solution is ________
(a)4.6\(\times\)103
(b)1.6\(\times\)104
(c)1\(\times\)10-5
(d)1\(\times\)105
-
Which one of the following names does not fit a real name?
(a)3 – Methyl –3–hexanone
(b)4–Methyl –3– hexanone
(c)3– Methyl –3– hexanol
(d)2– Methyl cyclo hexanone
-
Identify the colour formed in the test for phosphorous using ammonium molybdate.
(a)Crimson red colour
(b)Deep violet colour
(c)Prussian blue colour
(d)Canary yellow colour
-
The geometrical shape of carbocation is ______________.
(a)Linear
(b)tetrahedral
(c)Planar
(d)Pyramidal
-
Peroxide effect (Kharasch effect) can be studied in case of ___________
(a)Oct – 4 – ene
(b)hex – 3 – ene
(c)pent – 1 – ene
(d)but – 2 – ene
-
Which of the following is the most stable cycloalkane?
(a) (b)
(b) (c)
(c) (d)
(d)
-
Which of the following compounds will give racemic mixture on nucleophilic substitution by OH- ion?
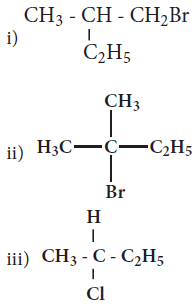 (a)
(a)(i)
(b)(ii) and (iii)
(c)(iii)
(d)(i) and (ii)
-
Calculate the molar mass of the following compounds.
Acetone [CH3 COCH3] -
After the execution of the \(\alpha\)-ray scattering experiment, what were the observations made by Rutherford? What did he conclude from his observations?
-
Name the first and the last elements in the following periods (n) .
(i) n=:4
(ii) n = 5
(iii) n = 6
(iv) n = 7 -
Write short notes on Deuterium.
-
Why alkaline earth metals are harder than alkali metals.
-
Distinguish between diffusion and effusion.
-
Explain intensive properties with two examples
-
A 0.25 M glucose solution at 370.28 K has approximately the pressure as blood does what is the osmotic pressure of blood ?
-
What is Grignard reagent? How is it prepared from ethyl bromide?
-
How much volume of chlorine is required to form 11.2 L of HCI at 273 K and 1 atm pressure?
-
Using Aufbau principle, write the ground state electronic configuration of following atoms.
(i) Boron (Z = 5)
(ii) Neon (Z = 10)
(iii) Aluminium (Z = 13)
(iv) Chlorine (Z = 17)
(v) Calcium (Z = 20)
(vi) Rubidium (Z = 37) -
Noble gases have maximum ionisation energy. Justify.
-
Why sodium hydroxide is much more water soluble than chloride ?
-
Would it be easier to drink water with a straw on the top of Mount Everest?
-
Identify processes under the following conditions
(i) dT = 0
(ii) dP = 0
(iii) dV = 0 -
The value of Kc for the reaction
N2O2(g) \(\rightleftharpoons \) 2NO2(g) -
0.16 g of an organic compound was heated in a carius tube and H2SO4 acid formed was precipitated with BaCl2. The mass of BaSO4 was 0.35g. Find the percentage of sulphur [30.04]
-
Write the equations for the preparation of l-iodobutane from.
-
-
Explain electron movement in organic reactions.
-
What happens when propene is treated with the following.
(i) H2SO4
(ii) Ozone
(iii) HBr
(iv) Red hot Fe tube
(v) Acidified KMnO4
-
-
-
Suggest and explain an indirect method to calculate lattice enthalpy of sodium chloride crystal
-
Explain the nature of non - ideal solution with positive deviation from Raoult,s law.
-
-
-
Dihydrogen reacts with dioxygen (O2) to form water. Write the name and formula of the product when the isotope of hydrogen which has one proton and one neutron in its nucleus is treated with oxygen. Will the reactivity of both the isotopes be the same towards oxygen? Justify your answer.
-
In an experimant of verification of Charle's law, the following are the set of readings taken by a student
Experiment Volume (L) Temperature (°C) 1 1.54 20 2 1.65 40 3 1.95 100 4 2.07 120 What is the average value of the constant of proportionality?
-
-
-
By using paulings method calculate the ionic radii of K+ and CI- ions in the potassium chloride crystal. Given that dk+-cl-=3.14 Å.
-
Give the uses of gypsum.
-
-
-
A laboratory analysis of an organic compound gives the following mass percentage composition: C = 60%, H = 4.48% and remaining oxygen.
-
What is the de Broglie wavelength (in cm) of a 160 g cricket ball travelling at 140 Km hr -1.
-
Part I
Answer all the questions.
Choose the most suitable answer
from the given four alternatives and write the option code with the
corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
6 x 2 = 12
Part III
Answer any 6 questions. Question no. 33 is compulsory.
6 x 3 = 18
Part IV
Answer all the questions.
5 x 5 = 25






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Chemistry - Half Yearly Model Question Paper 2019
Write your Comment