- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Chemistry Unit 11 Fundamentals of Organic Chemistry Book Back Questions Question Bank Software Sep-07 , 2019
Fundamentals of Organic Chemistry
Fundamentals of Organic Chemistry Book Back Questions
11th Standard
-
Reg.No. :
Chemistry
Time :
00:45:00 Hrs
Total Marks :
30
-
Select the molecule which has only one \(\pi\) bond.
(a)CH3– CH = CH – CH3
(b)CH3– CH = CH – CHO
(c)CH3– CH = CH – COOH
(d)All of these
-
In the hydrocarbo \(\overset { 7 }{ { CH }_{ 3 } } -\overset { 6 }{ { CH }_{ 2 } } -\overset { 5 }{ CH } =\overset { 4 }{ CH } -\overset { 3 }{ { CH }_{ 2 } } -\overset { 2 }{ C } =\overset { 1 }{ CH } \) the state of hybridisation of carbon 1,2,3,4 and 7 are in the following sequence.
(a)sp, sp, sp3, sp2, sp3
(b)sp2, sp, sp3, sp2, sp3
(c)sp, sp, sp2, sp, sp3
(d)none of these
-
Which one of the following names does not fit a real name?
(a)3 – Methyl –3–hexanone
(b)4–Methyl –3– hexanone
(c)3– Methyl –3– hexanol
(d)2– Methyl cyclo hexanone
-
IUPAC name of \({ CH }_{ 3 }-\overset { \underset { | }{ H } }{ \underset { \overset { | }{ { C }_{ 2 }{ H }_{ 5 } } }{ C } } -\overset { \underset { | }{ { C }_{ 4 }{ H }_{ 9 } } }{ \underset { \overset { | }{ { CH }_{ 3 } } }{ C } } -{ CH }_{ 3 }\) is ________
(a)3, 4, 4 – Trimethylheptane
(b)2 – Ethyl –3, 3– dimethyl heptane
(c)3, 4, 4 – Trimethyloctane
(d)2 – Butyl -2 –methyl – 3 – ethyl-butane
-
The IUPAC name of the compound
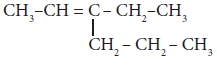 is ____________(a)
is ____________(a)3 – Ethyl -2– hexene
(b)3 – Propyl -3– hexene
(c)4 – Ethyl – 4 – hexene
(d)3 – Propyl -2-hexene
-
Give the general characteristics of organic compounds?
-
Write the molecular formula of the first six members of homologous series of nitro alkanes.
-
Describe the reactions involved in the detection of nitrogen in an organic compound by Lassaigne method.
-
Write all the possible isomers of molecular formula C4H10O and identify the isomerisms found in them.
-
0.40 g of an iodo-substituted organic compound gave 0.235 g of AgI by carius method. Calculate the percentage of iodine in the compound. (Ag = 108, I = 127)
-
0.33 g of an organic compound containing phosphorous gave 0.397 g of Mg2P2O7 by the analysis. Calculate the percentage of P in the compound.
-
0.26g of an organic compound gave 0.039 g of water and 0.245 g of carbon dioxide on combustion. Calculate the percentage of C & H.
-
0.185 g of an organic compound when treated with Conc. HNO3 and silver nitrate gave 0.320 g of silver bromide. Calculate the % of bromine in the compound. (Ag =108, Br = 80)
5 x 1 = 5
3 x 2 = 6
3 x 3 = 9
2 x 5 = 10






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Chemistry Unit 11 Fundamentals of Organic Chemistry Book Back Questions
Write your Comment