- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Five Question Bank Software Nov-10 , 2020
11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Five
11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Five
11th Standard
-
Reg.No. :
Chemistry
Time :
00:25:00 Hrs
Total Marks :
25
-
Atomicity of nitrogen is __________.
(a)1
(b)2
(c)3
(d)Zero
-
The maximum number of electrons in a sub shell is given by the expression _____________
(a)2n2
(b)2l + 1
(c)4l + 2
(d)none of these
-
Consider the following statements.
(i) The region where the probability density of electron is zero, called nodal surface.
(ii) The probability of finding the electron is independent of the direction of the nucleus.
(iii) The number of radial nodes is equal to n + 1+ 1
Which of the above statements is/are correct?(a)(i) and (iii)
(b)(i) and (ii)
(c)(iii) only
(d)(i) and (iii)
-
Pd has exceptional electronic configuration of 4d10 5s0 It belongs to period ______ and group _____
(a)4, 11
(b)5, 10
(c)6, 9
(d)3, 16
-
Find the incorrect statement.
(a)Smallest atom of periodic table is He
(b)p-block elements are metals, nonmetals and metalloids
(c)Noble gases have 8 valence electrons except He
(d)Valence electron and valency is same for group I
-
Which one of the following is the first transition series?
(a)Sc
(b)Zn
(c)Ti
(d)Cu
-
Which one of the following is not an isoelectronic ion?
(a)Na+
(b)Mg2+
(c)Cl-
(d)O2-
-
Among all the elements which one has the highest value of electronegativity?
(a)Chlorine
(b)Bromine
(c)Fluorine
(d)Iodine
-
Which of the following is arranged in order of increasing radius?
(a)K+ (aq) < Na+ (aq) < Li+ (aq)
(b)K+ (aq) > Na+ (aq) > Zn+ (aq)
(c)K+ (aq) > Li+ (aq) > Na+ (aq)
(d)Li+ (aq) < Na+ (aq) < K+ (aq)
-
The element with highest electron affinity belongs to___________.
(a)period 1 group 1
(b)period 3 group 17
(c)period 2 group 17
(d)period 2 group 16
-
Deuterium consist of _____________
(a)one electron, two proton, three neutron
(b)one electron, one proton, one neutron
(c)two electron, one proton, one neutron
(d)three electron, two proton, one neutron
-
The conversion of atomic hydrogen to dihydrogen is a __________ change.
(a)endothermic
(b)exothermic
(c)photochemical
(d)nuclear
-
Match the list Iwith List IIand select the correct answer using. the code given below the lists.
List I List II A H2O2 1 SiH4 B D2O 2 PdH C Metallic hydride 3 Bleach D Molecular hydride 4 Study of reaction mechanism (a)A B C D 1 3 2 4 (b)A B C D 4 3 1 2 (c)A B C D 3 4 2 1 (d)A B C D 2 1 4 3 -
H2O2 is a ________ acid.
(a)mono basic
(b)di basic
(c)tri basic
(d)none
-
Which of the following is used in illumination of wrist watches?
(a)Phosphorous
(b)Radon
(c)Tritium
(d)Deuterium
-
Metallic hydrides are otherwise called _______
(a)Salt hydrides
(b)Saline hydrides
(c)molecular hydrides
(d)Interstitial hydrides
-
Change in internal energy, when 4 kJ of work is done on the system and 1 kJ of heat is given out by the system is ______________
(a)+1 kJ
(b)- 5 kJ
(c)+3 kJ
(d)- 3 kJ
-
Consider the reaction where KP = 0.5 at a particular temperature
PCl5(g) ⇌ PCl3 (g) + Cl2 (g)
if the three gases are mixed in a container so that the partial pressure of each gas is initially 1 atm, then which one of the following is true ____________(a)more PCl3 will be produced
(b)more Cl2 will be produced
(c)more PCl5 will be produced
(d)none of these
-
The Henry's law constant for the solubility of Nitrogen gas in water at 350 K is 8 x 104 atm. The mole fraction of nitrogen in air is 0.5. The number of moles of Nitrogen from air dissolved in 10 moles of water at 350K and 4 atm pressure is ____________
(a)4 x 10-4
(b)4 x 104
(c)2 x 10-2
(d)2.5 x 10-4
-
Which of the following is electron deficient ?
(a)PH3
(b)(CH3)2
(c)BH3
(d)NH3
-
Non- Zero dipole moment is shown by __________
(a)CO2
(b)p-dichlorobenzene
(c)carbontetrachloride
(d)water
-
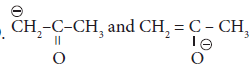 are ____________(a)
are ____________(a)resonating structure
(b)tautomers
(c)Optical isomers
(d)Conformers
-
The geometrical shape of carbocation is ______________.
(a)Linear
(b)tetrahedral
(c)Planar
(d)Pyramidal
-
Which of the following compounds will give racemic mixture on nucleophilic substitution by OH- ion?
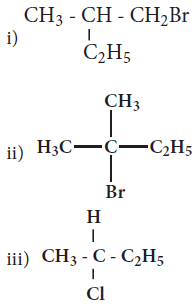 (a)
(a)(i)
(b)(ii) and (iii)
(c)(iii)
(d)(i) and (ii)
-
Haemoglobin of the blood forms carboxy haemoglobin with __________.
(a)Carbon dioxide
(b)Carbon tetra chloride
(c)Carbon monoxide
(d)Carbonic acid
Answer all the questions
25 x 1 = 25
*****************************************
Answers
-
(b)
2
-
(c)
4l + 2
-
(b)
(i) and (ii)
-
(b)
5, 10
-
(a)
Smallest atom of periodic table is He
-
(a)
Sc
-
(c)
Cl-
-
(c)
Fluorine
-
(d)
Li+ (aq) < Na+ (aq) < K+ (aq)
-
(c)
period 2 group 17
-
(b)
one electron, one proton, one neutron
-
(b)
exothermic
-
(c)
A B C D 3 4 2 1 -
(b)
di basic
-
(c)
Tritium
-
(d)
Interstitial hydrides
-
(c)
+3 kJ
-
(c)
more PCl5 will be produced
-
(d)
2.5 x 10-4
-
(c)
BH3
-
(d)
water
-
(b)
tautomers
-
(c)
Planar
-
(c)
(iii)
-
(c)
Carbon monoxide






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Five
Write your Comment