- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Nine Question Bank Software Nov-10 , 2020
11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Nine
11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Nine
11th Standard
-
Reg.No. :
Chemistry
Time :
00:25:00 Hrs
Total Marks :
25
-
Unit of Avogadro's number is _______________.
(a)mol
(b)g
(c)mol -1
(d)No unit
-
Consider the following statements and pick the incorrect statement(s).
1. Schrodinger wave equation is used to determine the probability of finding a electron at a given point in space.
2. The energy of a electron at infinity is positive
3. Angular momentum quantum number gives information regarding subshells.(a)1&3
(b)only 1
(c)only 2
(d)1,2 & 3
-
Which of the following process is important in food industry?
(a)Dehydration
(b)Dehalogenation
(c)Hydrogenation
(d)Carboxylation
-
In chelating method of softening of hard water_______________ is used
(a)magnesia
(b)lime
(c)EDTA
(d)washing soda
-
Which one of the following is known as Hydrogen sponge?
(a)Lithium hydride
(b)Diborane
(c)Palladium hydride
(d)Ammonia
-
Which of the following is a saline hydride?
(a)HCI
(b)NH3
(c)NaH
(d)PbH
-
Spodumene is the silicate mineral of _____
(a)lithium
(b)sodium
(c)cesium
(d)francium
-
Which of the following are stored under oil?
(a)Alkali metals
(b)Coinage metals
(c)Noble metals
(d)Phosphorous
-
Which of the following is the correct mathematical relation for Charles' law at constant pressure?
(a)\(V\propto T\)
(b)\(V\propto t\)
(c)\(V\propto \frac{1}{T}\)
(d)all of above
-
The standard free energy change ΔG0 is related to k (equilibrium constant) as ________
(a)ΔG0=RT log k
(b)ΔG0=RT log k
(c)ΔG0=-2.303 RT log k
(d)ΔG0=2.303 RT log k
-
Assertion (A): Spontaneous process is an irreversible process and may be reversed by some external agency.
Reason (R): Decrease in enthalpy is a contributory factor for spontaneity.
Codes:
(a) both A and R are true and R is the correct explanation of A
(b) both A and R are true and R is not correct explanation of A
(c) both A and R are false
(d) A is false but R is true -
A pressure cooker reduces cooking time for food because ___________
(a)Cooking involves chemical changes helped by a rise in temperature
(b)Heat is more evenly distributed in the cooking space
(c)Boiling point of water involved in cooking is increased
(d)The higher pressure inside the cooker crushes the food material
-
According to Valence bond theory, a bond between two atoms is formed when _____________
(a)fully filled atomic orbitals overlap
(b)half filled atomic orbitals overlap
(c)non- bonding atomic orbitals overlap
(d)empty atomic orbitals overlap
-
\(\underset { \underset { OH }{ | } }{ { CH }_{ 3 }-{ CH }_{ 2 }-{ CH }-CH_{ 2 }-{ CH }_{ 2 }-{ CH }_{ 3 } } \) and \(\underset { \underset { OH }{ | } }{ { CH }_{ 3 }-{ CH }_{ 2 }-{ CH_{ 2 } }-CH-{ CH }_{ 3 } } \) are ___________
(a)Functional isomers
(b)Position isomers
(c)Chain isomers
(d)These are not isomers
-
Which one of the following shows functional group isomerism?
(a)Ethene
(b)Acetone
(c)Ethane
(d)Propane
-
In \(C{ H }_{ 3 }-\underset { \overset { | }{ C{ H }_{ 3 } } }{ C } H-C{ H }_{ 3 }\) most stable radicals/ions formed on homolysis is/a __________
(a)\(C{ H }_{ 3 }-\underset { \overset { | }{ C{ H }_{ 3 } } }{ \overset { . }{ C } } H-C{ H }_{ 2 }\) and H
(b)\(C{ H }_{ 3 }-\underset { \overset { | }{ C{ H }_{ 3 } } }{ \overset { . }{ C } } H-C{ H }_{ 2 }\) and H
(c)\(C{ H }_{ 3 }-\underset { \overset { | }{ C{ H }_{ 3 } } }{ \overset { + }{ C } } -C{ H }_{ 3 }\) and H
(d)\(C{ H }_{ 3 }-\underset { \overset { | }{ C{ H }_{ 3 } } }{ \overset { - }{ C } } -C{ H }_{ 3 }\) and H
-
The most stable carbocation is ____________
(a)\(C{ H }_{ 3 }-\overset { + }{ C } { H }_{ 2 }\)
(b)\(C{ H }_{ 3 }-\overset { + }{ C } H-C{ H }_{ 3 }\)
(c)\(C{ H }_{ 2 }=CH-\overset { + }{ C } { H }_{ 2 }\)
(d)\(\overset { + }{ C } { H }_{ 3 }\)
-
The electrometric effect is ___________
(a)Permanent effect
(b)Temporary effect
(c)p-electrons transfer in the effect
(d)Both (b) and (c)
-
Consider the following compounds,
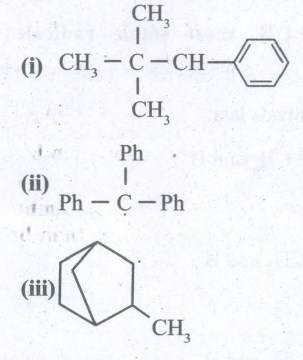
Hyper conjugation occurs in ____________(a)(i) and (iii)
(b)(i) only
(c)(ii) only
(d)(iii) only
-
Statement - I : All the organic molecules contain covalent bonds.
Statement - II : Organic molecules are formed by the mutual sharing of electrons between atoms.(a)Statement-I and II are correct and statement-Il is correct explanation of statement-I.
(b)Statement-I and II are correct but Statement-II is not correct explanation of statement-I.
(c)Statement-I is correct but statement-Il is wrong.
(d)Statement-I is wrong but statement-Il is correct.
-
Which one of the following is an example for negative mesomeric effect ?
(a)-SH
(b)-SR
(c)-NH2
(d)-NO2
-
Which of the following is aliphatic saturated hydrocarbon?
(a)C8 H18
(b)C9 H18
(c)C8 H14
(d)All of these
-
\({ CH }_{ 3 }-\overset { \overset { { CH }_{ 3 } }{ | } }{ CH } -{ CH }_{ 2 }-{ CH }3\overset { { CI }_{ 2 } }{ \underset { UV\quad light }{ \longrightarrow } } \underset { Major }{ (A) } ;\) the compound 'A' is ____________
(a)\({ CH }_{ 3 }-\overset { \underset { | }{ { CH }_{ 3 } } }{ CH } -\underset { \overset { | }{ CI } }{ { CH }_{ 2 } } -{ CH }_{ 3 }\)
(b)\({ CH }_{ 3 }-\overset { \underset { | }{ { CH }_{ 3 } } }{ \underset { \overset { | }{ CI } }{ C } } -{ CH }_{ 2 }-{ CH }_{ 3 }\)
(c)\({ CH }_{ 3 }-\overset { \underset { | }{ { CH }_{ 3 } } }{ CH } -{ CH }_{ 2 }-{ CH }_{ 2 }CI\)
(d)\({ CH }_{ 3 }-\overset { \underset { | }{ { CH }_{ 2 }CI } }{ \underset { \overset { | }{ H } }{ C } } -{ CH }_{ 2 }-{ CH }_{ 3 }\)
-
Select the correct statement
(a)Deviation from normal tetrahedral angle in cycloalkane is called angle stain
(b)Due to torsional stain eclipsed form has higher energy than the staggered form of a compound
(c)Chair form of cyclohexane is the most stable conformation of cyclohexane
(d)All of these
-
The cylindrical shape of alkyne is due to _________
(a)Two sigma C-C and one \(\pi\) C-C bonds
(b)One sigma C-C and two \(\pi\) C-C bonds
(c)Three sigma C-C bonds
(d)Three \(\pi\) C-C bonds
Answer all the questions
25 x 1 = 25
*****************************************
Answers
-
(d)
No unit
-
(c)
only 2
-
(c)
Hydrogenation
-
(c)
EDTA
-
(c)
Palladium hydride
-
(c)
NaH
-
(a)
lithium
-
(a)
Alkali metals
-
(a)
\(V\propto T\)
-
(c)
ΔG0=-2.303 RT log k
-
(c)
Boiling point of water involved in cooking is increased
-
(b)
half filled atomic orbitals overlap
-
(d)
These are not isomers
-
(b)
Acetone
-
(b)
\(C{ H }_{ 3 }-\underset { \overset { | }{ C{ H }_{ 3 } } }{ \overset { . }{ C } } H-C{ H }_{ 2 }\) and H
-
(c)
\(C{ H }_{ 2 }=CH-\overset { + }{ C } { H }_{ 2 }\)
-
(d)
Both (b) and (c)
-
(d)
(iii) only
-
(a)
Statement-I and II are correct and statement-Il is correct explanation of statement-I.
-
(d)
-NO2
-
(a)
C8 H18
-
(a)
\({ CH }_{ 3 }-\overset { \underset { | }{ { CH }_{ 3 } } }{ CH } -\underset { \overset { | }{ CI } }{ { CH }_{ 2 } } -{ CH }_{ 3 }\)
-
(d)
All of these
-
(b)
One sigma C-C and two \(\pi\) C-C bonds






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Nine
Write your Comment