- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Two Question Bank Software Nov-10 , 2020
11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Two
11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Two
11th Standard
-
Reg.No. :
Chemistry
Time :
00:25:00 Hrs
Total Marks :
25
-
7.5 g of a gas occupies a volume of 5.6 litres at 0° C and 1 atm pressure. The gas is ________.
(a)NO
(b)N2O
(c)CO
(d)CO2
-
Rusting of iron articles is an example of ___________ reaction
(a)Combustion
(b)decomposition
(c)redox
(d)hydrolysis
-
Which of the following is a mono-atomic molecule?
(a)Hydrogen
(b)Oxygen
(c)Sodium
(d)Ozone
-
The oxidation number of Cr in K2Cr2O7 is ___________.
(a)+4
(b)+6
(c)0
(d)+7
-
The number of nodes in s orbital of any energy level is equal to _________
(a)n
(b)2n2
(c)n-1
(d)n-2
-
Which of the following is the expected configuration of Cr (Z = 24)?
(a)1s2 2s2 2p6 3s2 3p6 3d4 4s2
(b)1s2 2s2 2p6 3s2 3p6 3d5 4s1
(c)1s2 2s2 2p6 3s2 3p6 3d6
(d)1s2 2s2 2p6 3s2 3p6 3d5 4s3
-
d-block elements form ____ compounds.
(a)ionic
(b)covalent
(c)Coordinate
(d)both (a) and (b)
-
In the following the element with the highest ionisation energy is _______
(a)[Ne]3s23p1
(b)[Ne]3s23p3
(c)[Ne]3s23p2
(d)[Ne]3s23p4
-
Consider the following statements.
(i) d-block elements show variable oxidate states.
(ii) Mostly d-block elements form colourless compounds.
(iii) Mostly d-block elements are diamagnetic due to paired electrons.
Which of the above statement is/are not correct?(a)(i) only
(b)(ii) only
(c)(i) and (ii)
(d)(ii) and (iii)
-
Which of the following possess almost same properties due to lanthanide contraction?
(a)Zr,HF
(b)Na,K
(c)Zn,Cd
(d)Ag,Au
-
Considering the elements F, CI, O and N, the correct order of their chemical reactivity in terms of oxidizing property is_____
(a)F > CI > O > N
(b)F > O > CI > N
(c)CI > F > O > N
(d)0 > F > N > CI
-
Consider the isoelectronic species Na+, Mg2+, F- and O2-. The correct order of increasing length of their radius is ______________
(a)F- < O2- < Mg2+< Na+
(b)Mg2+ < Na+ < F- < O2-
(c)O2- < F- < Na+ < Mg2+
(d)O2- < F- < Mg2+ < Na+
-
The element with highest electron affinity belongs to___________.
(a)period 1 group 1
(b)period 3 group 17
(c)period 2 group 17
(d)period 2 group 16
-
The number of neutrons in hydrogen atom is ___________.
(a)three
(b)two
(c)one
(d)zero
-
Hydrogen is used in _________
(a)hydrogenation of oils
(b)fuel cells
(c)gas bags for air ships
(d)all the above
-
Hydrogen accepts an electron to attain the inert gas configuration. In this way it resembles __________
(a)chalcogens
(b)halogens
(c)transition metals
(d)alkali metals
-
The maximum density of water is observed at ___________
(a)0oC
(b)4oC
(c)11.6oC
(d)273oC
-
CaO and NaCl have the same crystal structure and approximately the same radii. It U is the lattice energy of NaCl, the approximate lattice energy of CaO is _____________
(a)U
(b)2U
(c)U/2
(d)4U
-
The IUPAC name of
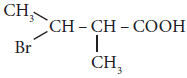 is ____________(a)
is ____________(a)2 – Bromo -3 – methyl butanoic acid
(b)2 - methyl - 3- bromobutanoic acid
(c)3 - Bromo - 2 - methylbutanoic acid
(d)3 - Bromo - 2, 3 - dimethyl propanoic acid
-
Homolytic fission of covalent bond leads to the formation of ___________.
(a)electrophile
(b)nucleophile
(c)Carbo cation
(d)free radical
-
The compounds formed at anode in the electrolysis of an aquous solution of potassium acetate are __________
(a)CH4 and H2
(b)CH4 and CO2
(c)C2H6 and CO2
(d)C2H4 and Cl2
-
Identify the compound 'Z' in the following reaction
\({ C }_{ 2 }{ H }_{ 6 }O\overset { { Al }_{ 2 }{ O }_{ 3 } }{ \underset { 623\quad K }{ \longrightarrow } } X\overset { { O }_{ 3 } }{ \longrightarrow } Y\overset { Zn/{ H }_{ 2 }O }{ \longrightarrow } \left( Z \right) \)(a)Formaldehyde
(b)Acetaldehyde
(c)Formic acid
(d)none of these
-
In the reaction
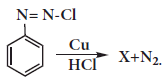 X is ________.(a)
X is ________.(a) (b)
(b) (c)
(c) (d)
(d)
-
Ethylidene chloride on treatment with aqueous KOH gives ___________
(a)acetaldehyde
(b)ehtyleneglycol
(c)formaldehyde
(d)glycoxal
-
Assertion (A) : Oxygen plays a key role in the troposphere
Reason (R) : Troposphere is not responsible for all biological activities
i) Both (A) and R are correct and (R) is the correct explanation of (A)
ii) Both (A) and R are correct and (R) is not the correct explanation of (A)
iii) Both (A) and R are not correct
iv) (A) is correct but( R) is not correct
Answer all the questions
25 x 1 = 25
*****************************************
Answers
-
(a)
NO
-
(c)
redox
-
(c)
Sodium
-
(b)
+6
-
(c)
n-1
-
(a)
1s2 2s2 2p6 3s2 3p6 3d4 4s2
-
(d)
both (a) and (b)
-
(b)
[Ne]3s23p3
-
(d)
(ii) and (iii)
-
(a)
Zr,HF
-
(b)
F > O > CI > N
-
(b)
Mg2+ < Na+ < F- < O2-
-
(c)
period 2 group 17
-
(d)
zero
-
(d)
all the above
-
(b)
halogens
-
(b)
4oC
-
(d)
4U
-
(c)
3 - Bromo - 2 - methylbutanoic acid
-
(d)
free radical
-
(c)
C2H6 and CO2
-
(a)
Formaldehyde
-
(b)
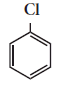
-
(a)
acetaldehyde






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium Free Online Test One Mark Questions with Answer Key 2020 - Part Two
Write your Comment