- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium Model Question Paper Part I Question Bank Software May-27 , 2020
11th Standard Chemistry English Medium Model Question Paper Part I
Model Question Paper Part I
11th Standard
-
Reg.No. :
Chemistry
Time :
02:30:00 Hrs
Total Marks :
70
-
12 g of carbon-12 contains_____carbon atoms
(a)6.022\(\times\)1023
(b)6
(c)12
(d)12.022\(\times\)10-23 kg
-
A macroscopic particle of mass 100 g and moving at a velocity of 100 cm S-1 will have a de Broglie wavelength of ___________
(a)6.6 x 10-29 cm
(b)6.6 x 10-30 cm
(c)6.6 x 10-31 cm
(d)6.6 x 10-32 cm
-
Electronegativity of the following elements increases in the order
(a)C, N, Si, P
(b)N, Si, C, P
(c)Si, P, C, N
(d)P,Si, N, C
-
Non-stoichiometric hydrides are formed by _____________
(a)palladium, vanadium
(b)carbon, nickel
(c)manganese, lithium
(d)nitrogen, chlorine
-
Match the list-I and list-Il using the correct code given below the list.
List-I List-II A Manufacture of soap 1 Na2CO3.10H2O B Mild antiseptic 2 Liquid Na metal C Softening of hard water 3 NaOH D Coolant in nuclear reactor 4 NaHCO3 (a)A B C D 4 3 2 1 (b)A B C D 3 4 1 2 (c)A B C D 2 1 3 4 (d)A B C D 1 2 4 3 -
The unit of pressure is _____________
(a)Pascal
(b)Torr
(c)Bar
(d)all the above
-
The bond dissociation energy of methane and ethane are 360 kJ mol-1 and 620 kJ mol-1 respectively. Then, the bond dissociation energy of C-C bond is ______________.
(a)170 kJ mol-1
(b)50 kJ mol-1
(c)80 kJ mol-1
(d)220 kJ mol-1
-
[Co(H2O)6]2+ (aq) (pink) + 4Cl– (aq) ⇌ [CoCl4]2– (aq) (blue) + 6 H2O (l)
In the above reaction at equilibrium, the reaction mixture is blue in colour at room temperature. On cooling this mixture, it becomes pink in colour. On the basis of this information, which one of the following is true?(a)ΔH > 0 for the forward reaction
(b)ΔH = 0 for the reverse reaction
(c)ΔH < 0 for the forward reaction
(d)Sign of the ΔH cannot be predicted based on this information
-
At same temperature, which pair of the following solutions are isotonic ?
(a)0.2 M BaCl2 and 0.2M urea
(b)0.1 M glucose and 0.2 M urea
(c)0.1 M NaCl and 0.1 M K2SO4
(d)0.1 M Ba (NO3)2 and 0.1 M Na2 SO4
-
Which molecule among the following has both polar and non-polar covalent bond?
(a)\({ NH }_{ 4 }^{ + }\)
(b)H2O2
(c)HCI
(d)CH4
-
The isomer of ethanol is ____________
(a)acetaldehyde
(b)dimethylether
(c)acetone
(d)methyl carbinol
-
Which one of the following is positively charged electrophiles ?
(a)CO2
(b)AlCl3
(c)BF3
(d)RX
-
In which of the following geometrical isomerism is possible?
(a)CH3CH = C(CH3)2
(b)C6H5N = NC6H5
(c)CH3CH = CH2
(d)All of these
-
Identify the correct order of boiling point of halo alkanes?
(a)CH3-CH2-CH2-CH2CI>(CH3)3C-CI > CH3-CH2-\(\underset { \overset { | }{ Cl } }{ CH } \)-CH3
(b)CH3-CH2-CH2-CH2CI>CH3-CH2-\(\underset { \overset { | }{ Cl } }{ CH } \)-CH3< (CH3)3C-CI
(c)(d) -
Assertion (A) : Oxygen plays a key role in the troposphere
Reason (R) : Troposphere is not responsible for all biological activities
i) Both (A) and R are correct and (R) is the correct explanation of (A)
ii) Both (A) and R are correct and (R) is not the correct explanation of (A)
iii) Both (A) and R are not correct
iv) (A) is correct but( R) is not correct -
Calculate the oxidation number of underlined atoms of the following:
NO3- -
How fast must a 54g tennis ball travel in order to have a de Broglie wavelength that is equal to that of a photon of green light 5400\(\overset { 0 }{ A } \) ?
-
Why alkaline earth metals are harder than alkali metals.
-
What are the applications of Charles' law?
-
Define molar heat of sublimation.
-
Write a balanced chemical equation for equilibrium reaction for which the equilibrium constant is given by expression
\(K_c={[NH_3]^4[O_2]^5\over [NO]^4[H_2O]^6}\) -
Write the favourable factors for the formation of ionic bond.
-
Give a brief description of the principles of
Fractional distillation -
Starting from CH3MgI, How will you prepare the following?
i) Acetic acid
ii) Acetone
iii) Ethyl acetate
iv) Iso propyl alcohol
v) Methyl cyanide. -
Calculate the oxidation number of underlined atoms \(H_{ 4 }\underline { { P }_{ 2 } } { O }_{ 7 }\)
-
Explain briefly the time independent schrodinger wave equation?
-
Justify that the fifth period of the periodic table should have 18 elements on the basis of quantum numbers.
-
Predict which of the following hydrides is a gas on a solid
(a) HCI
(b) NaH
Give your reason. -
Which would you expect to have a higher melting point magnesium oxide or magnesium fluoride ? Explain your reasoning.
-
8 g of methane is placed in a 5 litre container at 27° C. Find Boyle's constant.
-
Find out the value of equilibrium constant for the following reaction at 298K; 2NH3(g)+ CO2(g) \(\rightleftharpoons \) NH2CONH2(aq) + H2O(I) Standard Gibbs energy change, \(\Delta { G }_{ r }^{ 0 }\) at the given temperature is -13.6 kJ mol-1.
-
Vapour pressure of a pure liquid A is 10.0 torr at 27°C. The vapour pressure is lowered to 9.0 torr on dissolving one gram of B in 20 g of A. If the molar mass of A is 200 then calculate the molar mass of B.
-
Which of the following compounds will not exist as resonance hybrid? Give reason for your answer.
(i) CH3 - OH
(ii) R-CONH2
(iii) CH3-CH = CH-CH2NH2 -
Balancing of the molecular equation in alkaline medium.
MnO2 + O2 + KOH\(\rightarrow\)K2MnO4 + H2O -
Derive de-Broglie wave length.
-
(a) Define atomic radius.
(b) What are the difficulties in determining atomic radius? -
An isotope of hydrogen (A) reacts with diatomic molecule of element which occupies group number 16 and period number 2 to give compound (B) is used as a moderator in nuclear reaction. (A) adds on to a compound ( C), which has the molecular formula C3H6 to give (D). Identify A, B, C and D.
-
An element A belonging to group 2 and period 2 reacts with chlorine at an elevated temperature to give compound (B) compound B combines with LiAIH4 to form compound (C). Which is an hydride identify A, B, and C?
-
Show that the reaction \(CO+\frac { 1 }{ 2 } { O }_{ 2 }\longrightarrow { CO }_{ 2 }\) at 300K is spontaneous. The standard Gibbs free energies of formation of CO2 and CO are -394.4 and -137.2 KJ mole-1 respectively.
-
(i) What is meant by covalent bond ?
(ii) Explain the covalent bonding in H2, O2,Nr -
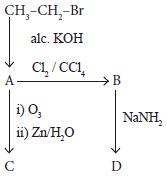
Identify the compound A, B, C and D in the following series of reactions
-
An organic compound Ⓐ of molecular formula C2H6O reacts with thionyl chloride in the presence of pyridine gives Ⓑ C2H5Cl. Ⓑ on reaction with alcoholic KOH gives ©, C2H4. ©️ on treatment with Cl2 gives C2H4Cl2 as Ⓓ. Identify Ⓐ,Ⓑ,Ⓒ,Ⓓ and explain the reaction.
-
How does classical smog differ from photochemical smog ?
Part I
Answer all the questions.
Choose the most suitable answer from the given four alternatives and write the option code with the corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
6 x 2 = 12
Part III
Answer any 6 questions. Question no. 33 is compulsory.
6 x 3 = 18
Part IV
Answer all the questions.
5 x 5 = 25






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium Model Question Paper Part I
Write your Comment