- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry English Medium Model Question Paper Part II Question Bank Software May-27 , 2020
11th Standard Chemistry English Medium Model Question Paper Part II
Model Question Paper Part II
11th Standard
-
Reg.No. :
Chemistry
Time :
02:30:00 Hrs
Total Marks :
100
-
The compound in which mass percentage of carbon is 75% and that of hydrogen is 25% is _____________.
(a)C2H6
(b)C2H2
(c)CH4
(d)C2H4
-
Based on equation E = \(-2.178\times { 10 }^{ -18 }J\left( \frac { { Z }^{ 2 } }{ { n }^{ 2 } } \right) \)certain conclusions are written. Which of them is not correct?
(a)Equation can be used to calculate the change in energy when the electron changes orbit
(b)For n = 1, the electron has a more negative energy than it does for n = 6 which means that the electron is more loosely bound in the smallest allowed orbit
(c)The negative sign in equation simply means that the energy of electron bound to the nucleus is lower than it would be if the electrons were at the infinite distance from the nucleus.
(d)Larger the value of n, the larger is the orbit radius.
-
The first list of 23 chemical elements was published by _____ in the year 1789.
(a)Berzelius
(b)Dobereiner
(c)Lavoisier
(d)John Dalton
-
Volume strength of 1.5 N H2O2 is _____________
(a)1.5
(b)4.5
(c)16.8
(d)8.4
-
The reducing power of a metal depends on various factors. Suggest the factor. which makes Li, the strongest reducing agent in aqueous solution __________
(a)Sublimation enthalpy
(b)Ionisation enthalpy
(c)Hydration enthalpy
(d)Electron-gain enthalpy
-
Pick out the correct relation for 1 mole of real gas.
(a)\(\left( P+\frac { V }{ { a }^{ 2 } } \right) (V-b)=RT\)
(b)\(P=\frac { RT }{ (V-b) } +\frac { a }{ { V }^{ 2 } } \)
(c)\(\left( P+\frac { a }{ { V }^{ 2 } } \right) (V-b)=RT\)
(d)\(\left( P-\frac { a }{ { V }^{ 2 } } \right) (V+b)=\frac { 1 }{ RT } \)
-
In an isothermal reversible compression of an ideal gas the sign of q, ΔS and w are respectively _______________
(a)+, -, -
(b)-, +, -
(c)+, -, +
(d)-, -, +
-
For the reaction AB (g) ⇌ A(g) + B(g), at equilibrium, AB is 20% dissociated at a total pressure of P, The equilibrium constant KP is related to the total pressure by the expression __________
(a)P = 24 KP
(b)P = 8 KP
(c)24 P = KP
(d)none of these
-
Assertion: An ideal solution obeys Raoults Law
Reason: In an ideal solution, solvent-solvent, as well as solute-solute interactions, are similar to solute-solvent interactions.
a) both assertion and reason are true and reason is the correct explanation of assertion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) both assertion and reason are false -
Which one of the following has pentagonal bipyramidal shape?
(a)XeF4
(b)XeOF4
(c)IF7
(d)IOF5
-
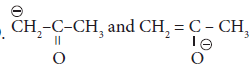 are ____________(a)
are ____________(a)resonating structure
(b)tautomers
(c)Optical isomers
(d)Conformers
-
Statement - I : Heterolytic cleavage is unsymmetrical one.
Statement- II : A covalent bond breaks and one of the bonded atom retains the bond pair of electrons.(a)Statement-I and II are correct and statement-II is correct explanation of statement-I.
(b)Statement-I and II are correct but statement-II is not correct explanation of statement-I.
(c)Statement-I is correct but statement-I! is wrong.
(d)Statement-I is wrong but statement-II is correct
-
Statement - I: Alkenes are more reactive than alkanes.
Statement -II: Because of the presence of a double bond.(a)Statement -I and II are correct and statement - II is correct explanation of statement - I
(b)Statement-I and II are correct but statement- II is not correct explanation of statement - I
(c)Statement - I is correct but statement - II is wrong.
(d)Statement - I is wrong but statement - II is correct.
-
Which ofthe following halogen exchange reaction will occur in acetone?
(a)R-I +NaCI
(b)R-F + KCI
(c)R-CI +NaI
(d)R- F +AgBrv
-
Assertion (A) : Excessive use of chlorinated pesticide causes soil and water pollution.
Reason (R) : Such pesticides are non-biodegradable.
i) Both (A) and R are correct and (R) is the correct explanation of (A)
ii) Both (A) and R are correct and (R) is not the correct explanation of (A)
iii) Both (A) and R are not correct
iv) (A) is correct but( R) is not correct -
Calculate the number of moles present in 60 g of ethane.
-
Which quantum number reveal information about the shape, energy, orientation and size of orbitals?
-
How is plaster of paris prepared ?
-
What is absolute zero? Mention its significance.
-
For the reaction, at 298K
2A + B ⟶ C
ΔH = 400 KJ mol-1, ΔS = 2 KJ mol-1
At what temperature will the reaction become spontaneous, considering, ΔH and ΔS to be constant over the temperature range? -
Write a balanced chemical equation for equilibrium reaction for which the equilibrium constant is given by expression
\(K_c={[NH_3]^4[O_2]^5\over [NO]^4[H_2O]^6}\) -
Explain the bond formation of hydrogen molecule.
-
0.30 g of a substance gives 0.88 g of carbon dioxide and 0.54 g of water calculate the percentage of carbon and hydrogen in it.
-
Explain the preparation of the following compounds
i) DDT
ii) Chloroform
iii) Biphenyl
iv) Chloropicrin
v) Freon-12 -
Calculate the oxidation number of nitrogen in nitrous acid and nitric acid
-
State and explain pauli exclusion principle.
-
In what period and group will an element with Z = 118 will be present?
-
Statues coated with white lead turn black on exposure to air. Its original colour is restored on treatment with H2O2 Explain.
-
Why sodium hydroxide is much more water soluble than chloride ?
-
What is compressibility factor? How does it explain the deviation of non ideal gases from ideal behaviour.
-
The equilibrium constant of a reaction is 10, what will be the sign of ΔG? Will this reaction be spontaneous?
-
If 5.6 g of KOH is present in
(a) 500 mL and
(b) 1 litre of solution
Calculate the molarity of each of these solutions. -
Carry over the following reaction mechanisms.
(i) Bromination of alkene
(ii) Addition of HCN to CH3CHO
(iii) Formation of alkyl bromide with benzoyl peroxide as radical initiator. -
An insecticide has the following percentage composition by mass: 47.5% C, 2.54% H, and 50.0% Cl. Determine its empirical formula and molecular formulae. Molar mass of the substance is 354.5g mol-1
-
Determine the following for the fourth shell of an atom.
(a) The number of subshells
(b) The designation for each subshell
(c) The number of orbitals in each subshell
(d) The maximum number of electrons that can be contained in each subshell -
Bring out the differences between electro negativity and electron affinity
-
A group-1 metal (A) which is present in common salt reacts with (B) to give compound (C) in which hydrogen is present in –1 oxidation state. (B) on reaction with a gas (C) to give universal solvent (D). The compound (D) on reacts with (A) to give (E), a strong base. Identify A, B, C, D and E. Explain the reactions.
-
State as to why
(a) Alkali metals show only +1 oxidation state.
(b) Na and K impart colour to the flame but Mg does not.
(c) Lithium on being heated in air mainly forms the monoxide and not the peroxide.
(d) Li is the best reducing agent in aqueous solution -
Calculate the entropy change when 1 mole of ethanol is evaporated at 351 K The molar heat of vaporisation of ethanol is 39.84 kJ mol-1.
-
What are the salient features of Valence Bond (VB) theory?
-
Give IUPAC names for the following compounds
CH3 – CH = CH – CH = CH – C ≡ C – CH3 -
Starting from methyl magnesium iodide how would you prepare
(i) Ethanol
(ii) 2-propanol
(iii) Tert-butyl alcohol -
Differentiate the following
(i) BOD and COD
(ii) Viable and non-viable particulate pollutants
Part I
Answer all the questions.
Choose the most suitable answer from the given four alternatives and write the option code with the corresponding answer.
15 x 1 = 15
Part II
Answer any 6 questions. Question no. 24 is compulsory.
6 x 2 = 12
Part III
Answer any 6 questions. Question no. 33 is compulsory.
6 x 3 = 18
Part IV
Answer all the questions.
5 x 5 = 25






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry English Medium Model Question Paper Part II
Write your Comment