- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students
Hydrocarbons Model Question Paper
11th Standard
-
Reg.No. :
Chemistry
Time :
02:00:00 Hrs
Total Marks :
50
-
Which of the following is optically active _________
(a)2 – methyl pentane
(b)citric acid
(c)Glycerol
(d)none of these
-
The compound that will react most readily with gaseous bromine has the formula (NEET)_____________
(a)C3H6
(b)C2H2
(c)C4H10
(d)C2H4
-
The major product formed when 2 – bromo – 2 – methyl butane is refluxed with ethanolic KOH is ___________
(a)2 – methylbut – 2 – ene
(b)2 – methyl butan – 1 – ol
(c)2 – methyl but – 1 – ene
(d)2 – methyl butan – 2 – ol
-
Some meta-directing substituents in aromatic substitution are given. Which one is most deactivating?
(a)– COOH
(b)– NO2
(c)– C ≡ N
(d)– SO3H
-
An alkane is obtained by decarboxylation of sodium propionate. Same alkane can be prepared by __________
(a)Catalytic hydrogenation of propene
(b)action of sodium metal on iodomethane
(c)reduction of 1 – chloro propane
(d)reduction of bromomethane
-
Sodalime is the mixture of_______
-
The potential energy difference between the staggered and eclipsed conformation of ethane is_________
-
Addition of hydrohalides to alkene is an example for________
-
Benzene contains_____ σ bonds and π bonds
-
Alkane
-
Alkyne
-
C4H10
-
C3H4
-
HDPE
-
How is propyne prepared from an alkyene dihalide?
-
Describe the mechanism of Nitration of benzene.
-
What happens when isobutylene is treated with acidified potassium permanganate?
-
Describe the conformers of n - butane.
-
Explain Markow nikoff's rule with suitable example.
-
Write the structures of folowing alkanes.
1) 2, 3 – Dimethyl – 6 – (2 – methyl propyl) decane
2) 5 – (2 – Ethyl butyl) – 3, 3 – dimethyldecane
3) 5 – (1, 2 – Dimethyl propyl) – 2 – methylnonane. -
How will you prepare propane from a sodium salt of fatty acid?
-
Listout the uses of alkanes.
-
Write a brief note on polymerisation of alkenes.
-
State Huckel's rule of aromaticity and explain it interms of cyclopentadiene, cycloxtateraene and cyclopropenylcation.
-
How to write the possible isomers of C5H12?
-
Identify the compound A, B, C and D in the following series of reactions
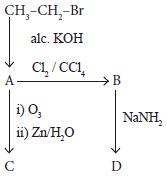
-
How are alkynes prepared from the following?
(i) Alkenes
(ii) Gem dihalides
(iii) Potassium maleate
(iv) Calcium carbide
5 x 1 = 5
4 x 1 = 4
5 x 1 = 5
(1)
2
(2)
Juice containers
(3)
CnH2n+2
(4)
1
(5)
CnH2n-2
7 x 2 = 14
4 x 3 = 12
2 x 5 = 10






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry - Hydrocarbons Model Question Paper
Write your Comment