- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

11th Standard Chemistry Public Exam March 2019 Important 5 Marks Questions Mar-13 , 2019
11th Public Exam March 2019 Important 5 Marks Questions
11th Public Exam March 2019 Important 5 Marks Questions
11th Standard
-
Reg.No. :
Chemistry
Time :
02:30:00 Hrs
Total Marks :
300
-
Calculate the molar mass of the following compounds.
i) urea [CO(NH2)2]
ii) Acetone [CH3 COCH3]
iii) Boric Acid [H3 BO3]
iv) Sulphuric Acid [H2 SO4] -
The reaction between aluminium and ferric oxide can generate temperatures up to 3273 K and is used in welding metals. (Atomic mass of AC = 27 u atomic mass of O = 16 u )
2Al + Fe2O3 \(\longrightarrow \) Al2O3 + 2Fe; If in this process, 324 g of aluminum is allowed to react with 1.12 kg of ferric oxide
i) Calculate the mass of Al2O3 formed
ii) How much of the excess reagent is left at the end of the reaction ? -
Write note on decomposition reaction
-
Balance the following equations by oxidation number method
i) \({ K }_{ 2 }{ Cr }_{ 2 }{ O }_{ 7 }+KI+{ H }_{ 2 }SO_{ 4 }\longrightarrow { K }_{ 2 }{ SO }_{ 4 }+{ Cr }_{ 2 }({ SO }_{ 4 })+{ I }_{ 2 }+{ H }_{ 2 }O\)
ii) \({ K }Mno_{ 4 }+{ Na }_{ 2 }{ So }_{ 3 }\longrightarrow { MnO }_{ 2 }+{ Na }_{ 2 }{ So }_{ 4 }+KOH\)
iii) \(Cu+{ HNO }_{ 3 }\longrightarrow Cu\left( { No }_{ 3 } \right) _{ 2 }+{ No }_{ 2 }+{ H }_{ 2 }O\)
iv) \({ KMn }O_{ 4 }+{ H }_{ 2 }{ C }_{ 2 }{ O }_{ 4 }+{ H }_{ 2 }{ SO }_{ 4 }\longrightarrow { K }_{ 2 }{ SO }_{ 4 }+{ MnSO }_{ 4 }+{ CO }_{ 2 }+{ H }_{ 2 }O\) -
Explain the term competitive electron transfer reaction with an example.
-
Balance the following equations by oxidation number method.
NH3 + F2 ⟶ HF + N2 -
Balance the following equation by ion-electron method In acidic medium.
\(Sb^{3+}{MnO}_4^{-} \rightarrow Sb^{5+}+Mn^{2+}\) -
A laboratory analysis of an organic compound gives the following mass percentage composition: C = 60%, H = 4.48% and remaining oxygen.
-
Enlist the postulates of Bohr's atom model.
-
Derive an equation for the wavelength of a matter wave.
-
The Li2+ ion is a hydrogen like ion that can be described by the Bohr model. Calculate the Bohr radius of the third orbit and calculate the energy of an electron in 4th orbit.
-
Suppose that the uncertainty in determining the position of an electron in an orbit is 0.6 \(\mathring{A}\) . What is the uncertainty in its momentum
-
The uncertainty in the position and velocity of a particle are 10-2 m and \(5.27\times { 10 }^{ -24 }{ ms }^{ -1 }\) respectively. Calculate the mass of the particle
-
Calculate the de Broglie wavelength of an electron that has been accelerated from rest through 1potential differences of 1 KV.
-
Calculate the uncertainty in the position of an electron, if the uncertainty in its velocity is 5.7 x 105 ms-1.
-
Derive de-Broglie wave length.
-
Explain the periodic trend of ionisation potential.
-
By using paulings method calculate the ionic radii of K+ and CI- ions in the potassium chloride crystal. Given that dk+-cl-=3.14 Å.
-
How do you classify of elements into blocks? Give their electronic configuration.
-
What are the factors which influence the electron gain enthalpy?
-
What are the factors influencing ionization enthalpy.
-
Explain about the anomalies of Mendeleev's periodic table.
-
A group-1 metal (A) which is present in common salt reacts with (B) to give compound (C) in which hydrogen is present in –1 oxidation state. (B) on reaction with a gas (C) to give universal solvent (D). The compound (D) on reacts with (A) to give (E), a strong base. Identify A, B, C, D and E. Explain the reactions.
-
Conc. H2SO4 cannot be used for drying hydrogen gas. Why?
-
Explain the exchange reactions of heavy water
-
Define hydrogen bond and its types.
-
Explain the action of water on
1) Na
2) Ba
3) Fe
4) Pb & Cu
5) Ag, Au,& Hg & Pt
6) C, S, & P -
Explain the important common features of Group 2 elements.
-
How are peroxides and superoxides formed by alkali metals?
-
Explain the preparation and uses of the following compounds of calcium.
-
Describe the method of electrolysis of brine solution?
-
Explain whether a gas approaches ideal behavior or deviates from ideal behaviour if
it is compressed to a smaller volume at constant temperature. -
Derive the ideal gas equation by combining the empirical gas laws.
-
Using Dalton's law how will you determine the pressure of a dry gas.
-
The vanderwaal's constants a = 2.095 lit2 atm mol-1 and b = 0.0189 lit mol-1 respectively. Calculate the inversion temperature.
-
Find the ratio of effusion rates of hydrogen and krypton gas.
-
At sea level a balloon has volume of 785 x10-3dm3 What will be its volume, if it taken to a place where the pressure is 0052 atm. Less than the atmospheric pressure of 1 atm.
-
A tank contains a mixture of 52.5 g of Oxygen and 65.1 g of CO2 at 300 K the total pressure in the tanks is 9.21 atm. calculate the partial pressure (in atm.) of each gas in the mixture.
-
Calculate the total pressure in a mixture of 8 g of oxygen and 4 g of hydrogen confined in a vessel of 1 dm3 at 27° C. [R = 0.083 bar dm3 K-1 mol-1.]
-
Define the following terms
(a) isothermal process (b) adiabatic process
(c) isobaric process (d) isochoric process -
Calculate the entropy change in the system, and surroundings, and the total entropy change in the universe during a process in which 245 J of heat flow out of the system at 77°C to the surrounding at 33°C.
-
For the reaction Ag2O(s) ⟶2Ag(s) + \(\frac{1}{2}\)O2(g) : ΔH =30.56 kJ mol-1 and AS = 6.66JK-1 mol-1 (at 1 atm). Calculate the temperature at which ΔG is equal to zero. Also predict the direction of the reaction (i) at this temperature and (ii) below this temperature.
-
A gas mixture of 3.67 lit of ethylene and methane on complete combustion at 25°C and at 1 atm pressure produce 6.11 lit of carbondioxide. Find out the amount of heat evolved in kJ, during this combustion. (ΔHc(CH4)= - 890 kJ mol-1 and (ΔHc(C2H4) = -1423 kJ mol-1
-
Calculate the standard entropy change for the following reaction (Δsf0), given the standard entropies of CO2(g) , C(s) 'O2(g) as 213.6, 5.740, and 205 JK-1 respectively.
-
In the reaction N2(g) + O2(g) ⟶ 2NO(g), ΔH0 reaction is 179.9 KJ mol-1 and ΔS0reaction=78.09 JK-1mol-1. Calculate ΔG0reaction at 300 K.
-
From the following data,
CH4+2O2 ➝ CO2+2H2O ΔHo= -890 KJ mol-1
H2O(l) ➝ H2O(g) ΔHo= 44 KJ mol-1 at 298 K
Calculate the enthalpy of the reaction
CH4+2O2 ➝ CO2+2H2O ΔHo=? -
Derive the various mathematical statements of the first law.
-
The atmospheric oxidation of NO
2NO(g) + O2(g) ⇌ 2NO2(g)
was studied with initial pressure of 1 atm of NO and 1 atm of O2. At equilibrium, partial pressure of oxygen is 0.52 atm calculate Kp of the reaction. -
The partial pressure of carbon dioxide in the reaction
CaCO3 (s) ⇌ CaO (s) + CO2(g) is 1.017 × 10–3 atm at 5000C. Calculate Kp at 6000C for the reaction. ΔH for the reaction is 181 KJ mol–1 and does not change in the given range of temperature. -
2.56 g of Sulphur is dissolved in 100g of carbon disulphide. The solution boils at 319. 692 K. What is the molecular formula of Sulphur in solution The boiling point of CS2 is 319. 450K. Given that Kb for CS2 = 2.42 K Kg mol-1.
-
Explain the nature of non - ideal solution with positive deviation from Raoult,s law.
-
Explain the various steps to draw the lewis structure of Nitirc acid.
-
How are bonding and anti-bonding molecular orbitals formed? Represent the constructive and destructive interaction in the 1s orbitals.
-
0.26g of an organic compound gave 0.039 g of water and 0.245 g of carbon dioxide on combustion. Calculate the percentage of C & H.
-
List down the characteristics possessed by the organic compounds.
-
How would you estimate the percentage of sulphur in an organic compound by Carius method?
-
Give a detailed account on homolytic and heterolytic cleavage.
-
Identify the compound A, B, C and D in the following series of reactions
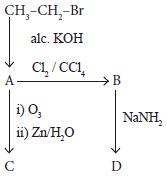
-
Ethane burns completely in air to give CO2, while in a limited supply of air gives CO. The same gases are found in automobile exhaust. Both CO and CO2 are atmospheric pollutants
i) What is the danger associated with these gases
ii) How do the pollutants affect the human body ? -
What are non-viable particulates ? How are they classified ? Explain.
60 x 5 = 300






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about 11th Standard Chemistry Public Exam March 2019 Important 5 Marks Questions
Write your Comment