- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

English medium Model question paper for chapter - 1 Basic Concepts of Chemistry and Chemical Calculations Apr-27 , 2019
Model question paper for Basic Concepts of Chemistry and Chemical Calculations
Model question paper for Basic Concepts of Chemistry and Chemical Calculations
11th Standard
-
Reg.No. :
Chemistry
Answer all questions
Time :
02:00:00 Hrs
Total Marks :
100
-
Rusting of iron articles is an example of ___________ reaction
(a)Combustion
(b)decomposition
(c)redox
(d)hydrolysis
-
Identify the correct statement(s) with respect to the following reaction :
Zn + 2HCl \(\longrightarrow\) ZnCl2 + H2
(i) Zinc is acting as an oxidant
(ii) Chlorine is acting as a reductant
(iii) Hydrogen is not acting as an oxidant
(iv) Zn is acting as a reductant(a)only (ii)
(b)only (iv)
(c)both (ii) and (iii)
(d)both (ii) and (i)
-
Match the list-I with list-II and select the correct answer using the code given below the lists.
List-I List-II A Cr2O72- 1 +5 B MnO4- 2 +6 C VO3- 3 +3 D FeF63+ 4 +7 (a)A B C D 3 1 4 2 (b)A B C D 4 3 2 1 (c)A B C D 2 4 1 3 (d)A B C D 3 2 1 4 -
Match the items in column list-I with relevant items in list-II.
List-I List-II A Ions having positive charge 1 anion B Ions having negative charge 2 -1 C Oxidation number of fluorine in NaF 3 0 D The sum of oxidation number of all atoms in a neutral molecule 4 cation (a)A B C D 3 4 2 1 (b)A B C D 1 2 3 4 (c)A B C D 2 3 4 1 (d)A B C D 4 1 2 3 -
Assertion (A): Among halogens fluorine is the best oxidant.
Reason (R): Fluorine is the most electronegative atom.
Codes:
(a) both assertion and reason are true and the reason is the correct explanation of assertion
(b) both assertion and reason are true but reason is not the correct explanation of assertion
(c) assertion is true but reason is false
(d) both assertion and reason are false -
Maximum oxidation state is present in the central metal atom of which compound
(a)CrO2Cl2
(b)MnO2
(c)[Fe(CN)6]3-
(d)MnO
-
The change in the oxidation number of S in H2S and SO2,in the following industrial reaction:
2H2S(g) + SO2(g) \(\longrightarrow\) 3S(s) + H2O(g)(a)-2 to 0, +4 to 0
(b)-2 to 0, +4 to -1
(c)-2 to -1, +4 to 0
(d)-2 to -1, +4 to -2
-
In which of the following reactions, hydrogen peroxide acts as an oxidising agent?
(a)I2+ H2O2 + 20H- \(\longrightarrow\) 21- + 2H2O + O2
(b)PbS + 4H2O2 \(\longrightarrow\) PbSO4 + 4H2O
(c)2MnO4-+ 3H2O2 \(\longrightarrow\) 2MnO2 + 3O2 + 2H2O + 2OH-
(d)HOCI + H2O2 \(\longrightarrow\) H2O+ + Cl- + O2
-
Identify the redox reaction taking place in a beaker.
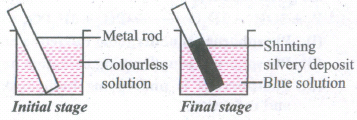 (a)
(a)Zn(s)+ Cu2+(aq) \(\longrightarrow\) Zn2+(aq) + Cu(s)
(b)Cu(s) + 2Ag+(aq) \(\longrightarrow\) Cu2+(aq) + 2Ag(s)
(c)Cu(s) + Zn2+(aq) \(\longrightarrow\) Zn(s) + cu2+ (aq)
(d)2Ag(s) + cu2+(aq) \(\longrightarrow\) 2Ag+aq + Cu(s)
-
Consider the following statements
i) Matter possesses mass.
ii) 22-carat gold is a mixture.
iii) Dry ice is a compound.
Which of the following statement(s) given above is/ are correct?(a)1 & 3
(b)Only 1
(c)1 & 2
(d)1,2, & 3
-
Match the list I with List II and select the correct answer using. the code given below the lists.
List I List II A Diamond 1 Heterogeneous mixture B Aerated drinks 2 Element C Distilled water 3 Homogeneous mixture D Sand 4 Compound (a)A B C D 2 3 4 1 (b)A B C D 4 3 1 2 (c)A B C D 3 1 4 2 (d)A B C D 2 1 4 3 -
The solid state of matter is converted into gas by
(a)sublimation
(b)deposition
(c)freezing
(d)condensation
-
The characteristic feature of orderly arrangement of molecules belongs to ______________.
(a)Solids
(b)Liquid
(c)Gases
(d)None of these
-
Which among the following statement(s) describe an element?
i) It is a pure substance which could be split into two or more simpler substance.
ii) It is a pure substance which cannot be split into simpler substance
iii) It's composition is not uniform
iv) All the above(a)only (iv)
(b)only (ii)
(c)(ii) and (iii)
(d)(i) and (iii)
-
Which form of based on physical characteristics possess neither definite volume nor definite shape?
(a)Solids
(b)Liquids
(c)Gases
(d)Both (a) and (b)
-
Calculate the molar mass of the following compounds.
Sulphuric Acid [H2 SO4] -
Balance the following equations by ion electron method
\(Zn+{ NO }_{ 3 }^{ - }\longrightarrow { Zn }^{ 2+ }+No\) -
Give a brief account of classification of matter.
-
Why atomic masses are called as relative atomic masses?
-
Give examples for the following redox reaction "Disproportionation."
-
Give the relationship between the number of mole of the substance and its gram molecular mass.
-
Calculate the equivalent masses of the following - HCl
-
Calculate the equivalent masses of the following - HNO3
-
Calculate the equivalent masses of the following - Oxalic acid H2C2O4
-
Calculate the equivalent masses of the following - Crystalline Oxalic acid, H2C2O4 , 2H2O
-
Calculate the equivalent masses of the following - Ferrous Sulphate
-
MnO42- undergoes disproportionation reaction in acidic medium but MnO4- does not. Give reason.
-
2Cu2S + 3O2 \(\longrightarrow\) 2Cu2O + 2SO2
(i) In this reaction which substance is getting oxidised and which substance is getting reduced?
(ii) Name the oxidising and reducing agents. -
How would you know whether a redox reaction is taking place in an acidic, alkaline or neutral medium?
-
What is the most essential conditions that must be satisfied in a redox reaction?
-
Zn rod is immersed in CuSO4 solution. What will you observe after an hour? Explain you observation in terms of the redox reaction.
-
Which one of the two, ClO2- or ClO4- shows disproportionation reaction and why?
-
'X' is an impure substance. Is it an element, compound or mixture?
-
Why is distilled water a compound whereas tap water is a mixture?
-
Why is air sometimes considered as a heterogeneous mixture?
-
By applying the knowledge of chemical classification, classify each of the following into elements, compounds or mixtures
Sugar -
Matter is defined as anything that has mass and occupies space. All matter is composed of atoms
-
Write a note on the differences between elements and compounds
-
How will you classify matter based on physical state ?
-
Explain the classification of matter based on chemical composition.
-
Calculate the number of atoms in the following 52 moles of He.
-
Calculate the mass of the following : 1 atom of silver
-
Calculate the mass of the following : 1 molecule of water.
-
How much mass (in gram units) is represented by the following ?
0.2 mol of NH3 -
Distinguish between the following.
(i) Atomic and molecular mass
(ii) Atomic mass and atomic weight
(iii) Empirical and molecular formula
(iv) Moles and molecules. -
In a reaction, A + B2 \(\longrightarrow \) AB2, identify the limiting reagent if any in the following reaction mixtures
(i) 300 atoms of A + 200 molecules of B
(ii) 2 moles of A + 3 moles of B
(iii) 100 atoms of A + 100 molecules of B
(iv) 5 moles of A + 2.5 moles of B
(v) 2.5 moles of A + 5 moles of B -
Balance the following equations by oxidation number method.
NH3 + F2 ⟶ HF + N2 -
Balance the following equations by oxidation number method.
K2Cr2O7 + FeSO4 + H2SO4 ⟶ K2SO4 + Cr2(SO4)3 + Fe2(SO4)3 + H2O -
Balance the following equation by oxidation number method.
C6H6 + O2\(\rightarrow\)CO2 + H2O -
Balance the following equation by oxidation number method.
KMnO4 + HCI \(\rightarrow\) KCl + MnCl2 + H2O + Cl2
Part - A
15 x 1 = 15
Part - B
Answer any 10 questions
10 x 2 = 20
Part - C
Answer any 15 questions 15 x 3 = 45
Part - D
Answer any 4 of the following questions
4 x 5 =20






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about English medium Model question paper for chapter - 1 Basic Concepts of Chemistry and Chemical Calculations
Write your Comment