- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Physics
-

Chemistry
-

Maths
-

Accountancy
-

Introductory Micro and Macroeconomics
-

Business Studies
-

Economics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Tamil
-

Bio Technology
-

Engineering Graphics
-

Entrepreneurship
-

Hindi Core
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Political Science
-

Psychology
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Mathematics
-

Chemistry
-

Biology
-

Physics
-

Business Studies
-

Accountancy
-

Economics
-

Computer Science
-

Bio Technology
-

English
-

Enterprenership
-

Geography
-

Hindi
-

History
-

Home Science
-

Physical Education
-

Political Science
-

Psychology
-

Sociology
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Mathematics
-

Social Science
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Science
-

Social Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Mathematics
-

Science
-

Social Science
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

Alkali And Alkaline Earth Metals & Gaseous State Important One Mark Question Paper In 11th Chemistry Sep-30 , 2018
Important 1mark -chapter 5,6
Important 1mark -chapter 5,6
11th Standard
-
Reg.No. :
Chemistry
Use blue pen Only
Time :
00:30:00 Hrs
Total Marks :
50
-
Which of the following statements is in correct ?
(a)Li+ has minimum degree of hydration among alkali metal cations
(b)The oxidation state of K in KO2 is +1
(c)Sodium is used to make Na / Pb alloy
(d)MgSO4 is readily soluble in water
-
Which of the following has the highest tendency to give the reaction \(M_{g}^{+}\xrightarrow[Medium]{Aqueous}M_{aq}^{+}\)
(a)Na
(b)Li
(c)Rb
(d)K
-
sodium is stored in _____________
(a)alcohol
(b)water
(c)kerosene
(d)none of these
-
RbO2 is _____________
(a)superoxide and paramagnetic
(b)peroxide and diamagnetic
(c)superoxide and diamagnetic
(d)peroxide and paramagnetic
-
Lithium shows diagonal relationship with _____________
(a)sodium
(b)magnesium
(c)calcium
(d)aluminium
-
In which process, fused sodium hydroxide is electrolysed for extraction of sodium ?
(a)Castner's process
(b)Cyanide process
(c)Down process
(d)All of these
-
Assertion : Generally alkali and alkaline earth metals form superoxides
Reason : There is a single bond between O and 0 in superoxides
a) both assertion and reason are true and reason is the correct explanation of assertion
b) both assertion and reason are true but reason is not the correct explanation of assertion
c) assertion is true but reason is false
d) both assertion and reason are false -
Which of the following statement is false ?
(a)Ca2+ ions are not important in maintaining the regular beating of the heart
(b)Mg2+ ions are important in the green parts of the plants
(c)Mg2+ ions form a complex with ATP
(d)Ca2+ ions are important in blood clotting
-
The name 'Blue John' is given to which of the following compounds ?
(a)CaH2
(b)CaF2
(c)Ca2(PO4)2
(d)CaO
-
When CaC2 is heated in atmospheric nitrogen in an electric furnace the compound formed is ___________
(a)Ca(CN)2
(b)CaNCN
(c)CaC2N2
(d)CaNC2
-
The order of decreasing ionisation enthalpy in alkali metals is _____________
(a)Na > Li > K > Rb
(b)Rb < Na < K < L
(c)Li > Na > K > Rb
(d)K < Li < Na < Rb
-
________ occurs in large amounts in sea water
(a)NaCI
(b)KCI
(c)both a and b
(d)neither a nor b
-
Name the alkaline earth metal hydroxide which is amphoteric is nature _________
(a)Be(OH)2
(b)KOH
(c)NaOH
(d)All of these
-
Gypsum is _________
(a)CaSO4.H2O
(b)CaSO4.1/2H2O
(c)CaSO4.1/4H2O
(d)CaSO4.2H2O
-
Which of the following statements is true about Ca(OH)2?
(a)It is used in the preparation of bleaching powder
(b)It is a light blue solid
(c)It is a light blue solid
(d)It is used in the manufacture of cement
-
By adding gypsum to cement ____________
(a)setting time of cement becomes less
(b)setting time Of cement increases
(c)colour of cement becomes light
(d)shining surface is obtained
-
Which is insoluble in water?
(a)CaF2
(b)CaCI
(c)HgCl2
(d)Ca(NO3)2
-
Which of the following is radioactive in group one elements?
(a)Lithium
(b)Caesium
(c)Rubidium
(d)Francium
-
Which of the following is insoluble in water?
(a)LiF
(b)NaCI
(c)KBr
(d)NaBr
-
In fire works, red colour flash is produced by ________
(a)Ba
(b)Ra
(c)Sr
(d)Rb
-
Which is used in dehydrating oils?
(a)Calcium
(b)Magnesium
(c)Beryllium
(d)Radium
-
Correctly match the list-I and list-II using the code given below the list
List-I List-II A Beryllium 1 Cement B Magnesium 2 Dating of rocks C Calcium 3 X-ray detector D Strontium 4 Missile construction (a)A B C D 3 4 1 2 (b)A B C D 4 3 2 1 (c)A B C D 1 4 3 2 (d)A B C D 2 3 4 1 -
Correctly match the list-land list-II using the code given below the list.
List-I List-II A Quick lime 1 Casts of statues B Calcium hydroxide 2 Drying agent C Gypsum 3 White washing D Plaster of paris 4 Tooth paste (a)A B C D 2 3 4 1 (b)A B C D 3 4 1 2 (c)A B C D 1 3 2 4 (d)A B C D 4 1 3 2 -
Which one of the following is named as bleaching powder?
(a)CaCI2
(b)CaOCI
(c)Ca(OCl)2
(d)Ca(HCO3)2
-
Statement-I: Cesium is considered as the most electropositive element.
Statement-Il: Due to its lowest ionization energy, cesium is considered as the most electropositive element.(a)Statements-I and II are correct and statement-II is the correct explanation of statement-I.
(b)Statements- I and II are correct but statement-II is not the correct explanation of statement- I.
(c)Statement-I is correct but statement-II is wrong.
(d)Statement-I is wrong but statement-II is correct.
-
Rate of diffusion of a gas is ________
(a)directly proportional to its density
(b)directly proportional to its molecular weight
(c)directly proportional to its square root of its molecular weight
(d)inversely proportional to the square root of its molecular weight
-
Which of the following is the correct expression for the equation of state of van der Waals gas?
(a)\(\left( P+\frac { a }{ { n }^{ 2 }{ V }^{ 2 } } \right) (V-nb)=nRT\)
(b)\(\left( P+\frac { na }{ { n }^{ 2 }{ V }^{ 2 } } \right) (V-nb)=nRT\)
(c)\(\left( P+\frac { { an }^{ 2 } }{ { V }^{ 2 } } \right) (V-nb)=nRT\)
(d)\(\left( \frac { P+{ n }^{ 2 }{ a }^{ 2 } }{ { V }^{ 2 } } \right) (V-ab)=nRT\)
-
When an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules _____________
(a)are above inversion temperature
(b)exert no attractive forces on each other
(c)do work equal to the loss in kinetic energy
(d)collide without loss of energy
-
The value of the gas constant R is ____________
(a)0.082 dm3 atm.
(b)0.987 cal mol-1K-1
(c)8.3 J mol-1 K-1
(d)8 erg mol-1 K-1
-
Equal moles of hydrogen and oxygen gases are placed in a container, with a pin-hole through which both can escape what fraction of oxygen escapes in the time required for one-half of the hydrogen to escape.
(a)\(\frac { 3 }{ 8 } \)
(b)\(\frac { 1 }{ 2 } \)
(c)\(\frac { 1 }{ 8 } \)
(d)\(\frac { 1 }{ 4 } \)
-
Four gases P, Q, R and S have almost same values of 'b' but their 'a' values (a, b are Vander Waals Constants) are in the order Q < R < S < P. At a particular temperature, among the four gases the most easily liquefiable one is __________
(a)P
(b)Q
(c)R
(d)S
-
Maximum deviation from ideal gas is expected from ______________
(a)CH4(g)
(b)NH3 (g)
(c)H2 (g)
(d)N2 (g)
-
Which of the following diagrams correctly describes the behaviour of a fixed mass of an ideal gas? (T is measured in K)
(a) (b)
(b) (c)
(c) (d)
(d)All of these
-
25g of each of the following gases are taken at 27°C and 600 mm Hg pressure. Which of these will have the least volume?
(a)HBr
(b)HCI
(c)HF
(d)HI
-
Value of gas constant R is ____________
(a)0.082dm3atm
(b)0.987 cal mol-1 K-1
(c)8.3 J mol-1 K-1
(d)8 er mol-1 K-1
-
The standard atmospheric pressure is the pressure that supports a column of mercury exactly ___________ high at 0° C at sea level.
(a)760mm
(b)76 cm
(c)both a & b
(d)760 cm
-
273K is equal to ______________ degree centigrade.
(a)0
(b)100
(c)373
(d)1
-
The liquefaction behaviour of temporary gases like CO2 approaches that of N2, O2 (permanent gases) as we go _______
(a)below critical temperature
(b)above critical temperature
(c)below absolute zero
(d)above absolute zero
-
Equal volumes of He, O2 and SO2 are taken in a closed container. The ratio of the partial pressures of gases He, O2 and SO2 would be __________
(a)1 : 2 : 8
(b)8 : 16 : 1
(c)1 : 4 : 16
(d)16 : 2 :1
-
The isotherm obtained for CO is as follows:
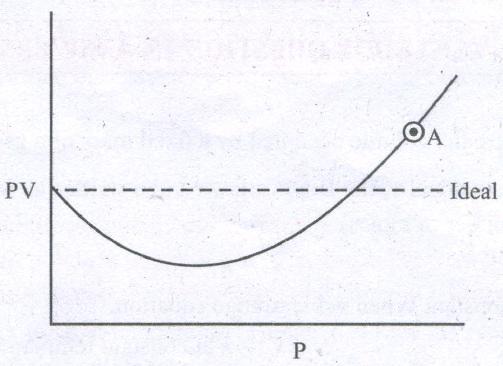
The compressibility factor for the gas at point ' A' will be __________(a)\((1-\frac{b}{V})\)
(b)\((1+\frac{b}{V})\)
(c)\((1+\frac{b}{RT})\)
(d)\((1+\frac{a}{RTV})\)
-
In Van der Waals equation of state for a non-ideal gas the net force of attraction among the molecules is given by _____________.
(a)\(\frac{an^2}{V^2}\)
(b)\(P+\frac{an^2}{V^2}\)
(c)\(P-\frac{an^2}{V^2}\)
(d)\(-\frac{an^2}{V^2}\)
-
Among the following, which is deadly poison?
(a)CO2
(b)HCN
(c)HCI
(d)NH3
-
Which one of the following is absolute zero?
(a)293 K
(b)273 K
(c)-273.15o C
(d)0o C
-
\(\frac{P}{T}\) = Constant is known as __________
(a)Boyle's law
(b)Charles' law
(c)Gay Lussac's law
(d)Dalton's law
-
The value of critical volume is equal in terms of Vander Waals constant is ___________.
(a)3b
(b)\(8a\over 27Rb\)
(c)\(a\over 27b^2\)
(d)\(2a\over Rb\)
-
The value of critical pressure of CO2 is ___________.
(a)173 atm
(b)73 atm
(c)1 atm
(d)22.4 atm
-
Statement-I: Gases do not liquefy above their critical temperature, even on applying high pressure.
Statement-II: Above critical temperature, the molecular speed is high and intermolecular attractions cannot hold the molecules together because they escape because of high speed.(a)Statement-I and II are correct and Statement-II is the correct explanation of Statement-I
(b)Statement-I and II are correct but Statement-Il is not the correct explanation of Statement-I
(c)Statement-I is correct but Statement-Il is wrong
(d)Statement-I is wrong but Statement-Il is correct
-
In a closed flask of 5 litres, 1.0 g of H2 is heated from 300 to 600 K, which statement is not correct?
(a)pressure of the gas increases
(b)the rate of the collusion increase
(c)the number of moles of gas increases
(d)the energy of gaseous molecules increases
Answer all the questions
50 x 1 = 50






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about Alkali And Alkaline Earth Metals & Gaseous State Important One Mark Question Paper In 11th Chemistry
Write your Comment