- State Board
-
12th Standard
-

Biology
-

Computer Applications
-

Computer Science
-

Business Maths and Statistics
-

Commerce
-

Economics
-

Maths
-

Chemistry
-

Physics
-

Computer Technology
-

History
-

Accountancy
-

Tamil
-

Maths
-

Chemistry
-

Physics
-

Biology
-

Computer Science
-

Business Maths and Statistics
-

Economics
-

Commerce
-

Accountancy
-

History
-

Computer Applications
-

Computer Technology
-

English
12th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
11th Standard
-

Maths
-

Biology
-

உயிரியல் - தாவரவியல்
-

Economics
-

Physics
-

Chemistry
-

History
-

Business Maths and Statistics
-

Computer Science
-

Accountancy
-

Commerce
-

Computer Applications
-

Computer Technology
-

Tamil
-

Maths
-

Commerce
-

Economics
-

Biology
-

Business Maths and Statistics
-

Accountancy
-

Computer Science
-

Physics
-

Chemistry
-

Computer Applications
-

History
-

Computer Technology
-

Tamil
-

English
11th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
9th Standard
-

-

-

-

-

-

-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
9th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
6th Standard
-

Maths
-

Science
-

Social Science
-

Maths
-

Science
-

Social Science
6th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
10th Standard
-

Maths
-

Science
-

Social Science
-

Tamil
-

Maths
-

Science
-

Social Science
-

English
-

English
10th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
7th Standard
-

Maths
-

Science
-

Maths
-

Science
-

Social Science
7th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
8th Standard
-

கணிதம் - old
-

Science
-

Social Science
-

கணிதம்
-

Maths
-

Science
-

Social Science
8th Standard stateboard question papers & Study material
தமிழ் Subjects
English Subjects
-
-
12th Standard
- CBSE Board
-
12th Standard CBSE
-

Biology
-

Chemistry
-

Physics
-

Maths
-

Accountancy
-

Business Studies
-

Economics
-

Introductory Micro and Macroeconomics
-

Computer Science
-

Geography
-

English
-

History
-

Indian Society
-

Physical Education
-

Sociology
-

Political Science
-

Engineering Graphics
-

Bio Technology
-

Entrepreneurship
-

Hindi Elective
-

Home Science
-

Legal Studies
-

Psychology
-

Hindi Core
-

Tamil
12th Standard CBSE Subject Question Paper & Study Material
-
-
11th Standard CBSE
-

Physics
-

Mathematics
-

Chemistry
-

Biology
-

Economics
-

Business Studies
-

Accountancy
-

Computer Science
-

English
-

Geography
-

History
-

Physical Education
-

Psychology
-

Sociology
-

Bio Technology
-

Enterprenership
-

Hindi
-

Home Science
-

Political Science
-

Applied Mathematics
11th Standard CBSE Subject Question Paper & Study Material
-
- 10th Standard CBSE
-
9th Standard CBSE
-

Social Science
-

Mathematics
-

Science
-

English
-

Hindi
9th Standard CBSE Subject Question Paper & Study Material
-
-
8th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
8th Standard CBSE Subject Question Paper & Study Material
-
-
7th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
7th Standard CBSE Subject Question Paper & Study Material
-
-
6th Standard CBSE
-

Social Science
-

Science
-

Mathematics
-

English
6th Standard CBSE Subject Question Paper & Study Material
-
-
12th Standard CBSE
- Free Online Test
- News
- Study Materials
-
Students
-

Stateboard Tamil Nadu
-

CBSE Board
-

Free Online Tests
-

Educational News
-

Scholarships
-

Entrance Exams India
-

Video Materials
Study Materials , News and Scholarships
-
-
Students

Alkali And Alkaline Earth Metals & Gaseous State Important Question Paper In 11th Chemistry Sep-30 , 2018
Important questions -chapter 5,6
Important questions -chapter 5,6
11th Standard
-
Reg.No. :
Chemistry
Use blue pen Only
Time :
01:00:00 Hrs
Total Marks :
50
-
Which of the following compounds will not evolve H2 gas on reaction with alkali metals ?
(a)ethanoic acid
(b)ethanol
(c)phenol
(d)none of these
-
A colourless solid substance (A) on heating evolved CO2 and also gave a white residue, soluble in water. Residue also gave CO2 when treated with dilute HCI ___________
(a)Na2CO3
(b)NaHCO3
(c)CaCO3
(d)Ca(HCO3)2
-
The general electronic configuration of alkali metals is ____________
(a)[noble gas] ns2
(b)[noble gas] ns1
(c)ns2 np6
(d)ns2 (n-1)d1-10
-
When an ideal gas undergoes unrestrained expansion, no cooling occurs because the molecules _____________
(a)are above inversion temperature
(b)exert no attractive forces on each other
(c)do work equal to the loss in kinetic energy
(d)collide without loss of energy
-
Equal moles of hydrogen and oxygen gases are placed in a container, with a pin-hole through which both can escape what fraction of oxygen escapes in the time required for one-half of the hydrogen to escape.
(a)\(\frac { 3 }{ 8 } \)
(b)\(\frac { 1 }{ 2 } \)
(c)\(\frac { 1 }{ 8 } \)
(d)\(\frac { 1 }{ 4 } \)
-
_____________ is the gas constant.
(a)a
(b)Vc
(c)R
(d)Tc
-
Pick out the correct relation for 1 mole of real gas.
(a)\(\left( P+\frac { V }{ { a }^{ 2 } } \right) (V-b)=RT\)
(b)\(P=\frac { RT }{ (V-b) } +\frac { a }{ { V }^{ 2 } } \)
(c)\(\left( P+\frac { a }{ { V }^{ 2 } } \right) (V-b)=RT\)
(d)\(\left( P-\frac { a }{ { V }^{ 2 } } \right) (V+b)=\frac { 1 }{ RT } \)
-
Statement I: At very high pressures, compressibility factor is greater than I.
Statement II: At very high pressure, 'b' can be neglected in vanderwaal's gas equation.(a)Both statement I and statement II are true and statement II explains statement I.
(b)Both statement I and statement II are true but statement II does not explain statement I
(c)Statement I is true but statement II is false.
(d)Both the statements are false.
-
Which of the following pairs of gases will diffuse at the same time through a porus plug?
(a)CO, NO2
(b)NO, C2H6
(c)NO2, CO2
(d)NH3,PH3
-
The value of Vander Waals constant "a" is maximum for __________.
(a)helium
(b)nitrogen
(c)methane
(d)ammonia
-
Mention the uses of plaster of paris
-
Beryllium halides are Covalent whereas magnesium halides are ionic why ?
-
What are the uses of sodium hydroxides?
-
Distinguish between diffusion and effusion.
-
Aerosol cans carry clear warning of heating of the can. Why?
-
Why sodium hydroxide is much more water soluble than chloride ?
-
Which would you expect to have a higher melting point magnesium oxide or magnesium fluoride ? Explain your reasoning.
-
Suggest why there is no hydrogen (H2) in our atmosphere. Why does the moon have no atmosphere?
-
In the below figure, let us find the missing parameters [volume in (b) and pressure in (c)]
P1 = 1 atm, P2 = 2 atm, P3 = ? atm
V1 = 1dm3, V2 =? dm3, V3 = 0.25 dm3
T1 = 298 K, T2 = 298 K, T3 = 298 K.
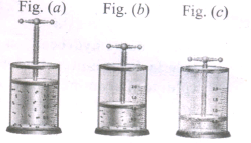
-
Explain Charles' law with an experimental illustration.
-
Discuss briefly the similarities between beryllium and aluminium.
-
Alkaline earth metal (A), belongs to 3rd period reacts with oxygen and nitrogen to form compound (B) and (C) respectively. It undergo metal displacement reaction with AgNO3 solution to form compound (D).
-
Defined the following terms.
-
75 ml of gas A effuses through a pin hole in 73 seconds the same volume of SO2 under identical conditions effuses in seconds. Calculate the molecular mass of A.
Part A
Answer all the questions
10 x 1 = 10
Part B
Answer all the questions
5 x 2 = 10
Part C
Answer all the questions
5 x 3 = 15
Part D
Answer all the questions
5 x 5 = 25






 11th Standard Chemistry Syllabus
11th Standard Chemistry Syllabus  11th Standard Chemistry Study Materials
11th Standard Chemistry Study Materials 11th Standard Chemistry MCQ Practise Tests
11th Standard Chemistry MCQ Practise Tests 

Reviews & Comments about Alkali And Alkaline Earth Metals & Gaseous State Important Question Paper In 11th Chemistry
Write your Comment